Exam 19: Ionic Equilibria in Aqueous Systems
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
What is the [H3O+] in a buffer that consists of 0.30 M HCOOH and 0.20 M HCOONa? For HCOOH, Ka = 1.7 × 10-4
(Multiple Choice)
4.9/5  (41)
(41)
A solution is prepared by adding 4.50 mol of sodium hydroxide to 1.00 L of 1.00 M Co(NO3)2. What is the equilibrium concentration of cobalt ions? Kf = 5.0 × 109 for Co(OH)42-
(Multiple Choice)
4.8/5  (27)
(27)
A buffer is prepared by adding 100 mL of 0.50 M sodium hydroxide to 100 mL of 0.75 M propanoic acid. Is this a buffer solution, and if so, what is its pH?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following indicators would be the best to use when 0.050 M benzoic acid (Ka = 6.6 × 10-5) is titrated with 0.05 M NaOH?
(Multiple Choice)
4.7/5  (38)
(38)
The solubility of silver chloride _______________ when dilute nitric is added to it.
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following substances has the greatest solubility in water?
(Multiple Choice)
4.9/5  (34)
(34)
A 20.0-mL sample of 0.25 M HNO3 is titrated with 0.15 M NaOH. What is the pH of the solution after 30.0 mL of NaOH have been added to the acid?
(Multiple Choice)
4.9/5  (32)
(32)
What is the pH of 375 mL of solution containing 0.150 mol of propenoic acid (HA) and 0.250 mol of sodium propenoate (NaA)? (Ka for propenoic acid is 5.52 × 10-5.)
(Short Answer)
4.8/5  (33)
(33)
When a strong acid is titrated with a weak base, the pH at the equivalence point
(Multiple Choice)
4.8/5  (39)
(39)
What is the pKa for the acid HA if a solution of 0.65 M HA and 0.85 M NaA has a pH of 4.75?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following aqueous mixtures would be a buffer system?
(Multiple Choice)
4.9/5  (28)
(28)
Use a carefully drawn and labeled diagram of the titration curve to illustrate the titration of a weak diprotic acid, in which Ka1 and Ka2 are substantially different, with a strong base (base is the titrant). Label as many features of the diagram as possible.
(Essay)
4.8/5  (44)
(44)
A 20.0-mL sample of 0.30 M HClO was titrated with 0.30 M NaOH. The following data were collected during the titration.  What is the Ka for HClO?
What is the Ka for HClO?
(Multiple Choice)
4.8/5  (35)
(35)
Consider the dissolution of MnS in water
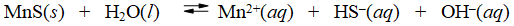 How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
How is the solubility of manganese(II) sulfide affected by the addition of aqueous potassium hydroxide to the system?
(Multiple Choice)
4.8/5  (32)
(32)
A 25.0-mL sample of 0.35 M HCOOH is titrated with 0.20 M KOH. What is the pH of the solution after 25.0 mL of KOH has been added to the acid? Ka = 1.77 × 10-4
(Multiple Choice)
4.9/5  (38)
(38)
Use the following information to calculate the solubility product constant, Ksp, for PbCl2. A saturated solution of PbCl2 in water was prepared and filtered. From the filtrate, 1.0 L was measured out into a beaker and evaporated to dryness. The solid PbCl2 residue recovered in the beaker amounted to 0.0162 moles.
(Multiple Choice)
4.8/5  (42)
(42)
What is the [H3O+] in a solution that consists of 1.5 M NH3 and 2.5 NH4Cl? Kb = 1.8 × 10-5
(Multiple Choice)
4.8/5  (39)
(39)
The salts X(NO3)2 and Y(NO3)2 (where X+ and Y+ are metal ions) are dissolved in water to give a solution which is 0.1 M in each of them. Which of the answers gives the concentration of chloride ions will precipitate the most YCl2 without precipitating any XCl2? Given Ksp values: XCl2, 2 × 10-5 YCl2, 1 × 10-10
(Multiple Choice)
4.8/5  (38)
(38)
A lab technician adds 0.015 mol of KOH to 1.00 L of 0.0010 M Ca(NO3)2. Ksp = 6.5 × 10-6 for Ca(OH)2). Which of the following statements is correct?
(Multiple Choice)
4.9/5  (42)
(42)
Assuming that the total volume does not change after 0.200 g of KCl is added to 1.0 L of a saturated aqueous solution of AgCl, calculate the number of moles of Ag+ ion in the solution after equilibrium has been reestablished. For AgCl, Ksp = 1.8 × 10- 10.
(Multiple Choice)
4.8/5  (35)
(35)
Showing 41 - 60 of 119
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)