Exam 2: The Chemical Basis of Life
Exam 1: Biology: Exploring Life47 Questions
Exam 2: The Chemical Basis of Life73 Questions
Exam 3: The Molecules of Cells89 Questions
Exam 4: A Tour of the Cell93 Questions
Exam 5: The Working Cell81 Questions
Exam 6: How Cells Harvest Chemical Energy82 Questions
Exam 7: Photosynthesis: Using Light to Make Food83 Questions
Exam 8: The Cellular Basis of Reproduction and Inheritance81 Questions
Exam 9: Patterns of Inheritance76 Questions
Exam 10: Molecular Biology of the Gene85 Questions
Exam 11: How Genes Are Controlled84 Questions
Exam 12: DNA Technology and Genomics80 Questions
Exam 13: How Populations Evolve67 Questions
Exam 14: The Origin of Species59 Questions
Exam 15: Tracing Evolutionary History88 Questions
Exam 16: Microbial Life: Prokaryotes and Protists80 Questions
Exam 17: The Evolution of Plant and Fungal Diversity85 Questions
Exam 18: The Evolution of Invertebrate Diversity81 Questions
Exam 19: The Evolution of Vertebrate Diversity77 Questions
Exam 20: Unifying Concepts of Animal Structure and Function68 Questions
Exam 21: Nutrition and Digestion96 Questions
Exam 22: Gas Exchange68 Questions
Exam 23: Circulation81 Questions
Exam 24: The Immune System76 Questions
Exam 25: Control of Body Temperature and Water Balance67 Questions
Exam 26: Hormones and the Endocrine System66 Questions
Exam 27: Reproduction and Embryonic Development88 Questions
Exam 28: Nervous Systems75 Questions
Exam 29: The Senses62 Questions
Exam 30: How Animals Move72 Questions
Exam 31: Plant Structure, Growth, and Reproduction81 Questions
Exam 32: Plant Nutrition and Transport69 Questions
Exam 33: Control Systems in Plants61 Questions
Exam 34: The Biosphere: an Introduction to Earths Diverse Environments61 Questions
Exam 35: Behavioral Adaptations to the Environment54 Questions
Exam 36: Population Ecology57 Questions
Exam 37: Communities and Ecosystems62 Questions
Exam 38: Conservation Biology61 Questions
Select questions type
Glycolysis is the first step of cellular respiration, in which glucose is used to generate ATP to power the cell. The major chemical reaction that takes place in glycolysis (ignoring some other reactants and products) is the conversion of glucose (C6H12O6) to pyruvate (C3H4O3) and hydrogen ions (H+). Using this information, what is the correct equation for the glycolysis chemical reaction?
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
B
Electrons move about the nucleus of an atom in the same way that
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
![Thin-layer chromatography is a method that can be used to separate molecules by their polarity. A mixture of molecules in a nonpolar liquid (the mobile phase) is added to the bottom of a piece of adsorbent material (the stationary phase) and allowed to migrate up the material. More-polar molecules interact more strongly with the stationary phase and thus migrate less. Less-polar molecules interact less strongly with the stationary phase and thus migrate more. Below are sample data for four different molecules from a thin-layer chromatography experiment. [Art: custom PowerPoint figure created by the author] -Below are the structures of two molecules: ethanol and cyclohexane. Which would migrate farther in a thin-layer chromatography experiment? [Art: modified from Wikipedia images of the structures]](https://storage.examlex.com/TB6039/11eaa8fa_4abf_69ab_96ab_1fc7cb4d03d0_TB6039_00_TB6039_00_TB6039_00.jpg) Thin-layer chromatography is a method that can be used to separate molecules by their polarity. A mixture of molecules in a nonpolar liquid (the mobile phase) is added to the bottom of a piece of adsorbent material (the stationary phase) and allowed to migrate up the material. More-polar molecules interact more strongly with the stationary phase and thus migrate less. Less-polar molecules interact less strongly with the stationary phase and thus migrate more. Below are sample data for four different molecules from a thin-layer chromatography experiment. [Art: custom PowerPoint figure created by the author]
-Below are the structures of two molecules: ethanol and cyclohexane. Which would migrate farther in a thin-layer chromatography experiment? [Art: modified from Wikipedia images of the structures]
Thin-layer chromatography is a method that can be used to separate molecules by their polarity. A mixture of molecules in a nonpolar liquid (the mobile phase) is added to the bottom of a piece of adsorbent material (the stationary phase) and allowed to migrate up the material. More-polar molecules interact more strongly with the stationary phase and thus migrate less. Less-polar molecules interact less strongly with the stationary phase and thus migrate more. Below are sample data for four different molecules from a thin-layer chromatography experiment. [Art: custom PowerPoint figure created by the author]
-Below are the structures of two molecules: ethanol and cyclohexane. Which would migrate farther in a thin-layer chromatography experiment? [Art: modified from Wikipedia images of the structures] ![Thin-layer chromatography is a method that can be used to separate molecules by their polarity. A mixture of molecules in a nonpolar liquid (the mobile phase) is added to the bottom of a piece of adsorbent material (the stationary phase) and allowed to migrate up the material. More-polar molecules interact more strongly with the stationary phase and thus migrate less. Less-polar molecules interact less strongly with the stationary phase and thus migrate more. Below are sample data for four different molecules from a thin-layer chromatography experiment. [Art: custom PowerPoint figure created by the author] -Below are the structures of two molecules: ethanol and cyclohexane. Which would migrate farther in a thin-layer chromatography experiment? [Art: modified from Wikipedia images of the structures]](https://storage.examlex.com/TB6039/11eaa8fa_4abf_dedc_96ab_a31f5e28717b_TB6039_00.jpg)
(Multiple Choice)
4.9/5  (27)
(27)
The sodium atom contains 11 electrons, 11 protons, and 12 neutrons. What is the mass number of sodium?
(Multiple Choice)
5.0/5  (26)
(26)
A diabetic, who does not utilize insulin properly, will metabolize fats instead of glucose. A condition called diabetic ketoacidosis is a common result of excessive fat metabolism, causing blood pH values of 7.1 or less (normal range is 7.35-7.45). What has happened to the blood pH and why?
(Multiple Choice)
4.7/5  (33)
(33)
The figure below shows five water molecules. The hydrogen bonds shown in this figure are each between 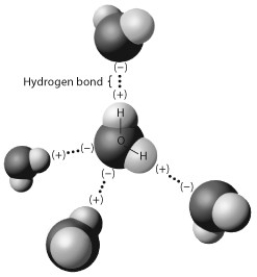
(Multiple Choice)
4.9/5  (32)
(32)
The body uses atoms in different ways to accomplish different tasks. For example, one portion of the body's calcium supply strengthens bones, whereas another portion combines with proteins to stimulate blood clotting after tissue injury. Which of the following statements provides the most logical chemical explanation of calcium's ability to perform such different functions?
(Multiple Choice)
4.8/5  (31)
(31)
You hypothesize that a tablet of PEPCID AC can neutralize more stomach acid than a tablet of Alka-Seltzer. If you placed a tablet of each antacid in a beaker of acid at an initial pH of 2, which of the following experimental results would support your hypothesis?
(Multiple Choice)
4.7/5  (45)
(45)
In the equation 2 H2 + O2 → 2 H2O, the H2 molecules are ________ and the H2O molecules are ________.
(Multiple Choice)
4.9/5  (26)
(26)
An uncharged atom of boron has an atomic number of 5 and an atomic mass of 11. How many electrons does boron have?
(Multiple Choice)
4.8/5  (39)
(39)
Uranium is a metallic element that is used in nuclear reactors and nuclear weapons. The vast majority of uranium found on Earth is in the form of uranium-238, an isotope with a mass number of 238, while the uranium that is used for nuclear reactors and weapons is uranium-235, an isotope with a mass number of 235.
-If the atomic mass of uranium is 92, how many neutrons does an atom of uranium-235 have in its nucleus?
(Multiple Choice)
4.8/5  (29)
(29)
The hydrogen atoms of a water molecule are bonded to the oxygen atom by ________ bonds, whereas neighboring water molecules are held together by ________ bonds.
(Multiple Choice)
4.9/5  (37)
(37)
You've been experiencing acid indigestion lately, and you'd like a quick fix for the problem. You do a little research on the Internet and discover that your problem is caused by excess stomach acid. In the pharmacy aisles, however, you're having a little trouble deciding what to purchase to address the problem. At the pharmacy counter, the clerk recommends that you purchase PEPCID AC or Alka-Seltzer tablets.
-If you could check the pH of the recommended tablets, you would expect it to be
(Multiple Choice)
4.9/5  (39)
(39)
In a water molecule, hydrogen and oxygen are held together by a ________ bond.
(Multiple Choice)
4.7/5  (40)
(40)
Below is the structure of octane, the major component of gasoline. What type(s) of bond(s) is(are) found in a molecule of octane? 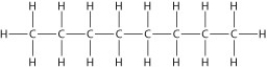
(Multiple Choice)
5.0/5  (28)
(28)
Showing 1 - 20 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)