Exam 17: Equilibrium in the Aqueous Phase
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
A cleaning solution has a pOH of 4.0. What is the pH and hydronium ion concentration of this solution?
(Essay)
4.8/5  (31)
(31)
The base ionization constant Kb describes which of the following reactions for a weak base, B, in aqueous solution?
(Multiple Choice)
4.8/5  (24)
(24)
A solution that contains a weak acid and its conjugate base in roughly equal concentrations is __________
(Multiple Choice)
4.8/5  (39)
(39)
A phosphate buffer solution (25.00 mL sample) used for a growth medium was titrated with 0.1000 M hydrochloric acid. The components of the buffer were sodium monohydrogenphosphate and sodium dihydrogenphosphate. The first endpoint occurred at a volume of 10.32 mL; the second occurred after an additional 18.62 mL was added (or total volume = 28.94 mL). What was the approximate ratio of HPO42- to H2PO4- in the buffer?
(Multiple Choice)
4.9/5  (38)
(38)
A solution with a pOH of 6.92 has an [OH-] concentration of __________
(Multiple Choice)
4.8/5  (33)
(33)
When an acetic acid solution is titrated with sodium hydroxide, the slope of the titration curve (pH vs volume of NaOH added) increases when sodium hydroxide is first added. This change shows that __________.
(Multiple Choice)
4.8/5  (33)
(33)
What are the characteristics of a pH indicator? A pH indicator __________
(I) is a weak acid.
(II) has different characteristic colors in protonated and unprotonated forms.
(III) changes color when the pH is near its pKa.
(Multiple Choice)
4.8/5  (48)
(48)
Write the reaction and equilibrium constant that describes the autoionization of water.
(Essay)
4.8/5  (34)
(34)
Carbon dioxide in the atmosphere dissolves in rainwater to make rain __________
(Multiple Choice)
4.8/5  (37)
(37)
Glycolic acid, which is a monoprotic acid and a constituent in sugar cane, has a pKa of 3.9. A 25.0 mL solution of glycolic acid is titrated to the stoichiometric point with 35.8 mL of 0.020 M sodium hydroxide solution. What is the pH of the resulting solution at the stoichiometric point?
(Multiple Choice)
4.9/5  (30)
(30)
Calculate the pH of a solution that is 0.30 M in ammonia (NH3) and 0.20 M in ammonium chloride
(NH4Cl, Ka = 5.62 10-10).
(Short Answer)
4.9/5  (36)
(36)
Magnesium sulfate can be obtained at a drugstore as Epsom salts. The monohydrate is found as the mineral kieserite. What would be the pH of a 500 mL aqueous solution containing 1.62 g kieserite? The 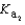 value for sulfuric acid is 1.2 10-2.
value for sulfuric acid is 1.2 10-2.
(Multiple Choice)
4.8/5  (41)
(41)
One brand of extra-strength antacid tablets contains 750 mg of calcium carbonate (100 g/mol) in each tablet. Stomach acid is essentially a hydrochloric acid solution. Is so much calcium carbonate really needed to neutralize stomach acid? Calculate the volume of stomach acid with a pH of 1.0 that one of these tablets could neutralize, and compare that value with the normal volume of stomach fluid, which usually is about 100 mL. One tablet can neutralize __________ mL of stomach acid at a pH of 1.0.
(Multiple Choice)
4.8/5  (38)
(38)
Use the following acid ionization constants to identify the correct decreasing order of base strengths. HF
Ka = 7.2 10-4
HNO2
Ka = 4.5 10-4
HCN
Ka = 6.2 10-10
(Multiple Choice)
4.9/5  (35)
(35)
At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL. The sharp rise is at 10.0 mL. 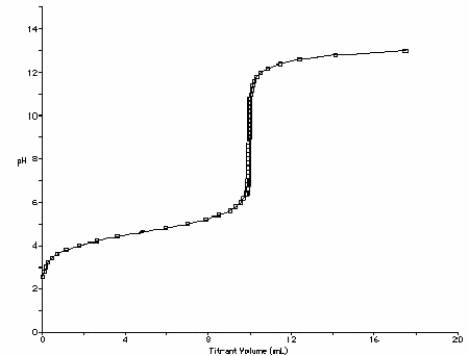
(Multiple Choice)
4.9/5  (38)
(38)
A buffer system is set up with [HA] = 2[A-]. If pKa = 5.5, what is the pH of the buffer?
(Multiple Choice)
4.9/5  (39)
(39)
What is the pH of a 0.15 M solution of ammonium bromide? The Kb value for ammonia is 1.8 10-5.
(Multiple Choice)
4.7/5  (41)
(41)
Which one of the following, A-D, is correct? If all are correct, respond E.
(Multiple Choice)
4.8/5  (35)
(35)
Showing 61 - 80 of 156
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)