Exam 17: Equilibrium in the Aqueous Phase
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O  H2PO4- + H3O+
H2PO4- + H3O+ 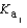 H2PO4- + H2O
H2PO4- + H2O 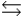 HPO42- + H3O+
HPO42- + H3O+ 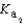 HPO42- + H2O
HPO42- + H2O 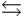 PO43- + H3O+
PO43- + H3O+ 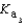 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following can be mixed together in water to produce a buffer solution?
(Multiple Choice)
4.9/5  (35)
(35)
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 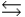 H2PO4- + H3O+
H2PO4- + H3O+ 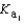 H2PO4- + H2O
H2PO4- + H2O 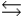 HPO42- + H3O+
HPO42- + H3O+ 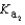 HPO42- + H2O
HPO42- + H2O 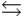 PO43- + H3O+
PO43- + H3O+ 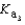 What is the Kb expression for the base, dihydrogen phosphate?
What is the Kb expression for the base, dihydrogen phosphate?
(Multiple Choice)
4.9/5  (45)
(45)
The acid ionization equilibrium constant, Ka, describes the reaction (where HA is a generic weak acid):
(Multiple Choice)
4.8/5  (33)
(33)
In the following reaction in aqueous solution, the acid reactant is __________ and its conjugate base product is __________. CH3COOH + NH3  CH3COO- + NH4+
CH3COO- + NH4+
(Multiple Choice)
4.7/5  (35)
(35)
The following titration curve is most likely to be associated with __________ 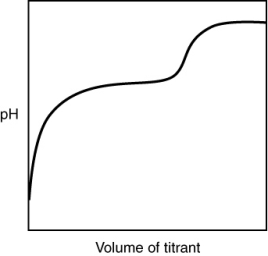
(Multiple Choice)
4.8/5  (38)
(38)
What is the pH of a 0.20 M solution of ammonia? The Kb value for ammonia is 1.8 10-5
(Multiple Choice)
4.8/5  (38)
(38)
Three acids found in foods are lactic acid (in milk products), oxalic acid (in rhubarb), and malic acid (in apples). The pKa values are LA = 3.88, OA = 1.23, and MA = 3.40. Which list has the conjugate bases of these acids in order of decreasing strength?
(Multiple Choice)
4.8/5  (30)
(30)
The first disinfectant used by Joseph Lister was called carbolic acid. This substance now is known as phenol, C6H5OH (pKa = 10.0). What is the pH of a 0.10 M solution of phenol?
(Multiple Choice)
4.8/5  (39)
(39)
The Yucca Mountain repository in Nevada, which is intended for the long-term storage of nuclear waste, has long been mired in controversy. One ongoing concern is whether the stainless-steel alloy containers could be corroded by salts such as calcium fluoride. If the calcium ion concentration in water inside Yucca Mountain is 1.25 10-3 M, what is the maximum possible concentration of fluoride ion in this water? Assume calcium fluoride Ksp = 2.2 10-10 at the temperatures inside the nuclear waste depository.
(Multiple Choice)
4.8/5  (40)
(40)
When values of Ka are small (e.g., 1 10-5) and concentrations of weak acids [HA] are relatively large (e.g., 0.10 M), the hydronium ion concentration of the solution can be calculated using which expression?
(Multiple Choice)
4.9/5  (42)
(42)
The pH of a popular soft drink is 3.4; what is its hydronium ion concentration?
(Multiple Choice)
5.0/5  (42)
(42)
The acidic ingredient in vinegar is acetic acid. The pH of vinegar is around 2.4, and the molar concentration of acetic acid in vinegar is around 0.85 M. Based on this information, determine the value of the acid ionization constant, Ka, for acetic acid.
(Multiple Choice)
4.9/5  (47)
(47)
Acid-base indicators need to have very intense colors so that a very low concentration is visible. Could there be a problem in using too much indicator?
(Multiple Choice)
4.7/5  (38)
(38)
Showing 41 - 60 of 156
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)