Exam 17: Equilibrium in the Aqueous Phase
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Explain how a buffer solution manages to stabilize the pH against the addition of acid, base, or additional solvent (dilution).
(Essay)
4.8/5  (35)
(35)
Which of the following is not a buffer system? A solution containing roughly equal concentrations of __________
(Multiple Choice)
4.9/5  (34)
(34)
The analysis label on a 500 mL bottle of Fiji Natural Artesian Water reports pH = 7.5 and 140 mg of bicarbonates, HCO3-. Calculate the pH expected as a result of the bicarbonates to see if these two numbers are consistent with each other. (Kb = 2.4 10-8 for HCO3-)
(Multiple Choice)
4.8/5  (33)
(33)
The concentration of acetic acid (pKa = 4.75) in vinegar is about 1.0 M. With this information, what do you predict the pH of vinegar to be?
(Short Answer)
4.8/5  (42)
(42)
Identify all the correct statements about an acid-base buffer solution.
(I) It can be prepared by combining a strong acid with a salt of its conjugate base.
(II) It can be prepared by combining a weak acid with a salt of its conjugate base.
(III) It can be prepared by combining a weak base with its conjugate acid.
(IV) The pH of a buffer solution does not change when the solution is diluted.
(V) A buffer solution resists changes in its pH when an acid or base is added to it.
(Multiple Choice)
4.7/5  (38)
(38)
In a titration of monoprotic acids and bases, there is a large change in pH __________
(Multiple Choice)
4.8/5  (44)
(44)
A mining operation needs to separate silver and copper. You are hired as a summer intern in a beautiful Colorado mining town, and one of your tasks is to determine whether this separation can be accomplished by oxidizing the metals to Ag+ and Cu+, each one at a concentration of 0.15 M, and then precipitate one as the chloride salt without precipitate the other. AgCl precipitates first. What is the concentration of the Ag+ remaining in solution when CuCl begins to precipitate? For AgCl, pKsp = 9.74. and for CuCl, pKsp = 6.76.
(Multiple Choice)
4.9/5  (42)
(42)
What is the pH of a solution made from 46.20 mL of a 0.0500 nitric acid solution diluted to 100.0 mL?
(Short Answer)
4.7/5  (42)
(42)
Which expression defines the autoionization constant for water, Kw?
(Multiple Choice)
4.8/5  (30)
(30)
Which ending to the statement is not correct? The weak acid HY will be stronger than the weak acid HZ if __________
(Multiple Choice)
4.8/5  (44)
(44)
What is the hydronium ion concentration of a 0.20 M solution of ammonia? The Kb value for ammonia is 1.8 10-5
(Multiple Choice)
4.8/5  (39)
(39)
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 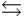 H2PO4- + H3O+ Ka
H2PO4- + H3O+ Ka
 H2PO4- + H2O
H2PO4- + H2O 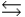 HPO42- + H3O+ Ka
HPO42- + H3O+ Ka
 HPO42- + H2O
HPO42- + H2O  PO43- + H3O+ Ka
PO43- + H3O+ Ka
 What is the Kb expression for the base, sodium phosphate?
What is the Kb expression for the base, sodium phosphate?
(Multiple Choice)
4.8/5  (43)
(43)
The concentration of acetic acid in vinegar is about 1.0 M, and the pH is about 2.4. With this information, what do you expect the pKa of vinegar to be?
(Short Answer)
4.8/5  (35)
(35)
What is the pOH of a 0.20 M solution of ammonia? The Kb value for ammonia is 1.8 10-5
(Multiple Choice)
4.9/5  (48)
(48)
In each of the following, the stronger acid is identified and an explanation is given. All except one are correct statements. Which one is not correct?
(Multiple Choice)
4.9/5  (47)
(47)
Which sketch best represents the qualitative molecular view of an aqueous solution of nitric acid? (Water molecules are not shown explicitly.)
(Multiple Choice)
4.9/5  (35)
(35)
Four solutions have pH values of 3, 5, 8, and 10. Arrange these in order of increasing acidity, and identify those that are acidic, basic, and neutral.
(Essay)
4.7/5  (38)
(38)
Which sketch best represents the qualitative molecular view of an aqueous solution of nitrous acid? (Water molecules are not shown explicitly.)
(Multiple Choice)
4.8/5  (30)
(30)
What is the solubility of barium sulfate in a solution containing 0.050 M sodium sulfate? The Ksp value for barium sulfate is 1.1 10-10.
(Multiple Choice)
4.9/5  (36)
(36)
Showing 21 - 40 of 156
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)