Exam 17: Equilibrium in the Aqueous Phase
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
The most suitable acid-base indicator for a titration of acetic acid with NaOH has __________
(Multiple Choice)
4.8/5  (29)
(29)
Three acids found in foods are lactic acid (in milk products), oxalic acid (in rhubarb), and malic acid (in apples). The pKa values are LA = 3.88, OA = 1.23, and MA = 3.40. Which list has these acids in order of decreasing acid strength?
(Multiple Choice)
4.8/5  (34)
(34)
In the Brønsted-Lowry definition of acids and bases, an acid __________
(Multiple Choice)
4.9/5  (43)
(43)
What is the actual concentration of molecular NH3 in a 0.200 M solution of ammonia? The Kb value for ammonia is 1.80 10-5.
(Multiple Choice)
4.8/5  (36)
(36)
The solubility product for an insoluble salt with the formula MX3 is written as __________, where s is the molar solubility.
(Multiple Choice)
4.9/5  (33)
(33)
Stalactites-the long, icicle-like formations that hang from the ceilings of caves-are formed from recrystallizing minerals like calcite (calcium carbonate). The Ksp of calcium carbonate is 4.5 10-9. What is the concentration of a saturated calcium carbonate solution?
(Multiple Choice)
4.8/5  (41)
(41)
Explain why some salts produce neutral solutions while others produce solutions that are acidic or basic, and give an example for each.
(Essay)
4.9/5  (43)
(43)
What would happen to the Ag+ and Cl- concentrations if NaCl(s) was added to a saturated solution of AgCl in water?
(Multiple Choice)
4.7/5  (34)
(34)
Which one of the following is not a conjugate acid-base pair?
(Multiple Choice)
4.8/5  (27)
(27)
What is the pH of a 0.25 M NH4Cl solution (Ka = 5.68 10-10)?
(Short Answer)
4.9/5  (40)
(40)
Phenylephrine (PE, see the structure below) is a nasal decongestant and is the active ingredient in Sudafed, which contains phenylephrine hydrochloride (PEHCl). This conjugate acid of phenylephrine (PEH+) has a pKa = 5.5. At a physiological pH of 7.4. what is the ratio of concentrations, [PE]/[PEH+]? ![Phenylephrine (PE, see the structure below) is a nasal decongestant and is the active ingredient in Sudafed, which contains phenylephrine hydrochloride (PEHCl). This conjugate acid of phenylephrine (PEH<sup>+</sup>) has a pK<sub>a </sub>= 5.5. At a physiological pH of 7.4. what is the ratio of concentrations, [PE]/[PEH<sup>+</sup>]?](https://storage.examlex.com/TB3833/11eaae02_0891_77f6_95d8_93f83ee99a85_TB3833_00.jpg)
(Multiple Choice)
4.8/5  (36)
(36)
A solution of sulfuric acid (H2SO4, 25.00 mL) was titrated to completion with 34.55 mL of 0.1020 M sodium hydroxide. What was the concentration of the sulfuric acid?
(Multiple Choice)
4.8/5  (29)
(29)
Vitamin C is a monoprotic weak acid, which also is called ascorbic acid (C6H8O5, 176 g/mol). A vitamin C tablet weighing 0.75 g was dissolved in 50.0 mL of water and titrated with 0.250 M sodium hydroxide. It took 12.5 mL of the NaOH solution to reach the end point. What is the percentage of vitamin C in the tablet? Only the vitamin C reacted with the NaOH.
(Multiple Choice)
4.7/5  (46)
(46)
The solubility of PbBr2 is 0.427 g per 100 mL of solution at 25°C. Determine the value of the solubility product constant for this strong electrolyte. Lead(II) bromide does not react with water.
(Multiple Choice)
4.8/5  (31)
(31)
The following titration curve is most likely to be associated with 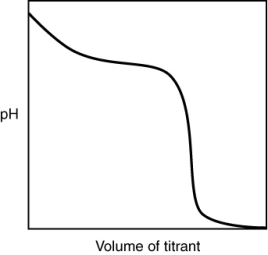
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following would be the best choice for preparing a buffer with a pH = 8.0?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following compounds cannot be a Brønsted-Lowry base?
(Multiple Choice)
4.9/5  (33)
(33)
Showing 121 - 140 of 156
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)