Exam 17: Equilibrium in the Aqueous Phase
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
When sodium chloride is added to a saturated solution of lead(II) chloride, some of the lead(II) chloride precipitates. This phenomenon is called __________
(Multiple Choice)
4.8/5  (37)
(37)
Which one of the following would make the best buffer? (Ac = acetate)
(Multiple Choice)
4.9/5  (40)
(40)
The concentration of acetic acid (pKa = 4.75) in vinegar is about 1.0 M. With this information, what do you predict the pH of vinegar to be?
(Multiple Choice)
4.9/5  (41)
(41)
Sometimes liquid ammonia, NH3, is used as a solvent rather than water. Which expression defines the ammonia autoionization counterpart of Kw?
(Multiple Choice)
4.8/5  (46)
(46)
The solubility product for an insoluble salt with the formula M3X is written as __________, where s is the molar solubility.
(Multiple Choice)
4.9/5  (38)
(38)
As the pH decreases, the solubility of __________ would increase.
(Multiple Choice)
4.9/5  (30)
(30)
Stalactites-the long, icicle-like formations that hang from the ceilings of caves-are formed from recrystallizing minerals like calcite (calcium carbonate). The Ksp of calcium carbonate is 4.5 10-9. What is the minimum volume of dripping water saturated with calcium carbonate that would be required to form a small stalactite that had a mass of 1.000 kg?
(Multiple Choice)
4.7/5  (39)
(39)
Which one of the following salts does not produce a basic solution when dissolved in water?
(Multiple Choice)
4.9/5  (44)
(44)
A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution. Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution. The resulting titration curves are illustrated here. Given the following possibilities, what is the sample? 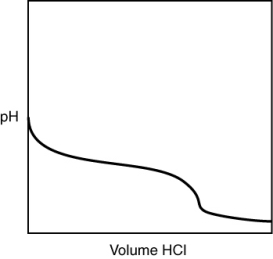
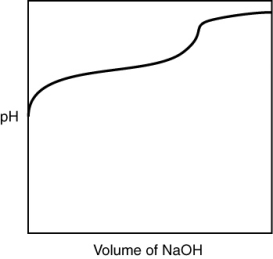
(Multiple Choice)
4.8/5  (40)
(40)
Halfway to the equivalence point in a titration curve of a weak acid with a strong base, __________
(Multiple Choice)
4.8/5  (40)
(40)
The solubility product for an insoluble salt with the formula MX2 is written as __________, where s is the molar solubility.
(Multiple Choice)
4.8/5  (42)
(42)
A 0.100 M solution of a monoprotic weak acid has a pH of 3.00. What is the pKa of this acid?
(Multiple Choice)
4.9/5  (40)
(40)
Bert and Ernie were determining the pH of their goldfish's water. Bert's pH meter was in the fix-it shop, so Ernie used his pOH meter instead. Ernie insists that he can use the reading to calculate the pH by simple addition or subtraction that he learned in second grade, but Bert (showing off as usual) claims that the calculation involves finding an antilogarithm, dividing, and then finding a logarithm. This would make use of all the math he learned in third grade. Who is right?
(Multiple Choice)
5.0/5  (34)
(34)
The pH of an aqueous sodium fluoride (NaF) solution is __________ because __________
(Multiple Choice)
4.8/5  (33)
(33)
Derive the Henderson-Hasselbalch equation from the acid ionization constant expression.
(Essay)
4.9/5  (30)
(30)
Which one of the following salts forms aqueous solutions with pH = 7?
(Multiple Choice)
4.9/5  (39)
(39)
A phosphate buffer solution (25.00 mL sample) used for a growth medium was titrated with 0.1000 M hydrochloric acid. The components of the buffer were sodium monohydrogenphosphate and sodium dihydrogenphosphate. The first endpoint occurred at a volume of 10.32 mL, and the second occurred after an additional 18.62 mL was added, for a total volume of 28.94 mL. What was the total concentration of phosphate (in any form) in the buffer?
(Multiple Choice)
4.8/5  (33)
(33)
In the following reaction in aqueous solution, the acid reactant is __________, and its conjugate base product is __________. CH3NH2 + HSO4- 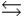 CH3NH3+ + SO42-
CH3NH3+ + SO42-
(Multiple Choice)
4.8/5  (36)
(36)
The solubility product for an insoluble salt with the formula M2X is written as __________, where s is the molar solubility.
(Multiple Choice)
4.7/5  (37)
(37)
Showing 101 - 120 of 156
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)