Exam 8: Reactions of Alkenes
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 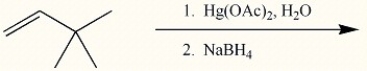
Free
(Essay)
4.9/5  (39)
(39)
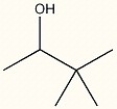
Correct Answer:
Give the structure of the alkene which would yield the following products upon ozonolysis-reduction.
CH3CH2CH2CH2CHO + CH2O
Free
(Essay)
5.0/5  (38)
(38)
Correct Answer:
CH3CH2CH2CH2CH  CH2
CH2
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
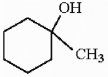
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 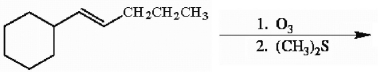
(Essay)
4.9/5  (32)
(32)
Provide the reagents necessary to complete the following transformation. 
(Essay)
4.8/5  (37)
(37)
Consider how the I-Cl bond is polarized and predict the product which results when this mixed halogen adds to 1-methylcyclohexene.
(Essay)
4.7/5  (40)
(40)
Which of the following steps would successfully complete the following reaction? 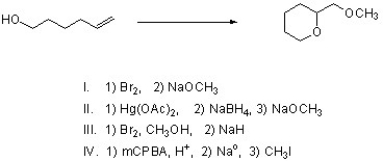
(Multiple Choice)
4.9/5  (32)
(32)
An unknown compound with empirical formula C3H5 was treated with Br2/CCl4. The bromine solution went from orangish/red to clear immediately at room temperature. Upon treatment with O3 followed by work-up with dimethylsulfide the following products were identified. From the information provided what is/are the most likely structure(s) for this unknown compound. 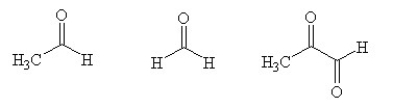
(Multiple Choice)
4.7/5  (35)
(35)
Give the structure of the alkene which would yield the following products upon ozonolysis-reduction.
CH3COCH3 + CH3CH2CHO
(Essay)
4.7/5  (38)
(38)
Provide the reagents necessary to complete the following transformation. 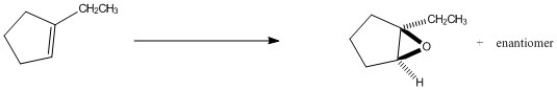
(Multiple Choice)
4.8/5  (33)
(33)
Using any alkene as your starting material, how could you make the alkyl halide shown? 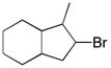
(Essay)
4.9/5  (36)
(36)
Treatment of cyclopentene with peroxybenzoic acid ________.
(Multiple Choice)
4.9/5  (35)
(35)
The mechanism for the acid-catalyzed hydration of alkenes is simply the reverse of the mechanism by which alcohols are dehydrated using concentrated acid. This is an illustration of the principle of ________.
(Short Answer)
4.8/5  (29)
(29)
When propylene reacts with hydrogen bromide in the presence of a peroxide initiator, which of the following structures are formed during the mechanism?
(Multiple Choice)
4.9/5  (33)
(33)
Draw the major organic product generated in the reaction below. Pay particular attention to regio- and stereochemical detail. 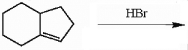
(Essay)
4.8/5  (43)
(43)
Provide a detailed, step-by-step mechanism for the reaction shown below. 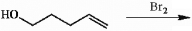
(Essay)
4.8/5  (37)
(37)
Provide the reagents necessary to complete the following transformation. 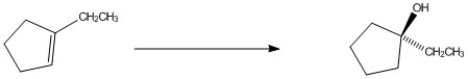
(Multiple Choice)
4.9/5  (38)
(38)
Showing 1 - 20 of 134
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)