Exam 25: Lipids
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
What occurs when an aqueous solution of sodium stearate is treated with a solution of calcium ions?
Free
(Essay)
4.9/5  (42)
(42)
Correct Answer:
Calcium stearate, an insoluble salt, precipitates.
Provide the major organic product of the following reaction sequence. 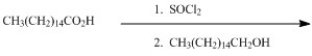
Free
(Essay)
5.0/5  (26)
(26)
Correct Answer:
CH3(CH2)14CO2CH2(CH2)14CH3
Which of the following terms best describes the compound below? 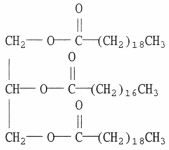
Free
(Multiple Choice)
5.0/5  (33)
(33)
Correct Answer:
B
Which of the following fatty acids has the highest melting point?
(Multiple Choice)
4.9/5  (39)
(39)
The following natural product has been examined for cytotoxic activity. How should this natural product be classified? 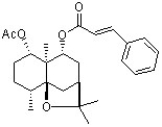
(Multiple Choice)
4.7/5  (44)
(44)
If triolein (oleic acid: CH3(CH2)7CH  CH(CH2)7COOH) was reacted with hydrogen in the presence of a transition metal catalyst, what would the new product be and how would the properties of the new compound be different from triolein?
CH(CH2)7COOH) was reacted with hydrogen in the presence of a transition metal catalyst, what would the new product be and how would the properties of the new compound be different from triolein?
(Essay)
4.8/5  (23)
(23)
What reagent is needed to convert CH3(CH2)14CO2H to CH3(CH2)14CH2OH?
(Short Answer)
4.9/5  (37)
(37)
Which of the following structural features is not typically found in a prostaglandin?
(Multiple Choice)
4.8/5  (38)
(38)
What does the term polyunsaturated mean when used to describe a triglyceride?
(Essay)
4.8/5  (32)
(32)
Lauric acid has the formula CH3(CH2)10CO2H. Provide the structure of the triglyceride which was formed from three equivalents of lauric acid.
(Essay)
4.8/5  (39)
(39)
Draw the structure of the product which results when the compound shown below is completely hydrogenated using excess H2. 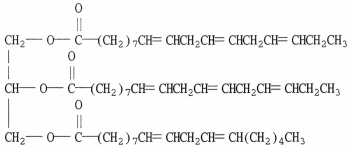
(Essay)
4.8/5  (37)
(37)
Was the compound shown below more likely isolated from an animal or a plant? Explain. 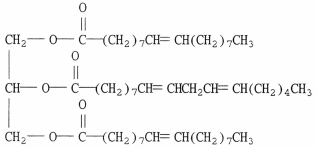
(Essay)
4.8/5  (29)
(29)
When spermaceti is heated in aqueous potassium hydroxide, 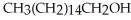 and
and 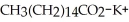 are formed. Provide the structure of spermaceti.
are formed. Provide the structure of spermaceti.
(Essay)
4.8/5  (39)
(39)
Oleic acid is an example of ________ fatty acid. A molecule of oleic acid contains a single carbon-carbon double bond and ________ carbon atoms.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following terms best describes the interior of a soap micelle in water?
(Multiple Choice)
4.8/5  (25)
(25)
Showing 1 - 20 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)