Exam 11: Reactions of Alcohols
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
The enzyme, alcohol dehydrogenase, which is produced by the liver, converts ethanol to ________, a normal body metabolite.
Free
(Short Answer)
4.9/5  (33)
(33)
Correct Answer:
acetic acid
What series of synthetic steps could be used to prepare PhCH(OH)CH2CH3 from 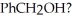
Free
(Essay)
4.9/5  (40)
(40)
Correct Answer:
1) PCC
2) CH3CH2MgBr
3) H3O+
How could tell a sample of n-butanol from tert-butanol?
Free
(Essay)
4.8/5  (33)
(33)
Correct Answer:
Use the Lucas test. When adding the reagents, a second phase would form immediately with tert-butanol while it would be much slower with n-butanol.
Predict the major product of the reaction below and provide a stepwise mechanism which accounts for its formation.
CH3(CH2)6CH2OH + HBr →
(Essay)
4.7/5  (33)
(33)
How can the electrophilicity of hydroxyls be increased? Suggest several specific ways.
(Essay)
4.8/5  (34)
(34)
Provide the structure of the major organic product in the reaction below. 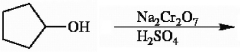
(Essay)
4.7/5  (31)
(31)
Given a sample of (R)-2,3-dimethylhexan-3-ol, which of the following would be the best synthesis of (R)-3-chloro-2,3-dimethylhexane?
(Multiple Choice)
4.8/5  (35)
(35)
Which reagent could be used to perform the following reaction? 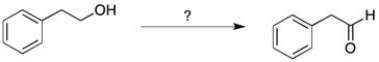
(Multiple Choice)
4.8/5  (36)
(36)
When (R)-butan-2-ol is treated with TsCl in pyridine, the product formed is ________.
(Multiple Choice)
4.8/5  (31)
(31)
Provide the structure of the major organic product in the reaction below. 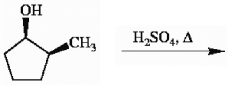
(Essay)
4.9/5  (31)
(31)
What series of synthetic steps could be used to prepare 1-bromopentane from 1-bromopropane?
(Essay)
4.8/5  (39)
(39)
What is the best way to make the ether shown below using a Williamson Ether Synthesis reaction? 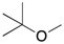
(Multiple Choice)
4.8/5  (30)
(30)
Provide the structure of the major organic product in the reaction below. 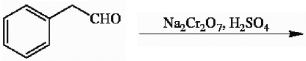
(Essay)
4.8/5  (36)
(36)
What type of linkage bonds the individual nucleotides together in DNA?
(Short Answer)
4.8/5  (38)
(38)
Name the alcohol that would be used in the Fischer esterification synthesis of ethyl propanoate (CH3CH2O2CCH2CH3).
(Short Answer)
4.8/5  (30)
(30)
Provide the structure of the major organic product that results when 1-octanol is treated with pyridinium chlorochromate.
(Essay)
4.8/5  (27)
(27)
Dimethyl ether (CH3OCH3) can be prepared by heating methanol in concentrated H2SO4. Provide a detailed, stepwise mechanism for this process.
(Essay)
4.7/5  (37)
(37)
Showing 1 - 20 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)