Exam 5: Stereochemistry
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Is the molecule shown below chiral or achiral? 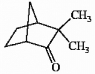
Free
(Short Answer)
4.9/5  (35)
(35)
Correct Answer:
chiral
Which of the following terms best describes the pair of compounds shown: enantiomers, diastereomers, or the same compound? 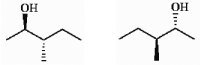
Free
(Short Answer)
4.8/5  (36)
(36)
Correct Answer:
the same compound
Label each asymmetric carbon in the compound below as R or S. 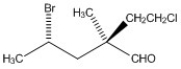
Free
(Essay)
4.9/5  (39)
(39)
Correct Answer:
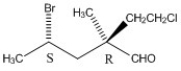
Can one separate a mixture of enantiomers by gas chromatography? Explain.
(Essay)
4.9/5  (36)
(36)
Which of the statements below correctly describes an achiral molecule?
(Multiple Choice)
4.8/5  (32)
(32)
Can the two compounds be separated by distillation? Why or why not?
(1S,2S,3R,5S)-Pinanediol and (1S,2R,3R,5R)-Pinanediol
(Essay)
4.8/5  (32)
(32)
Assign the proper configurational label, R or S, to the chiral carbon in the molecule shown below. 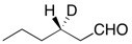
(Short Answer)
4.8/5  (43)
(43)
If possible, draw the structure of the enantiomer of the molecule shown below. 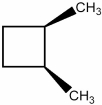
(Essay)
4.8/5  (35)
(35)
The stereochemical configuration of D-Threonine is (2R, 3S). Which of the following structures corresponds to the correct configuration? 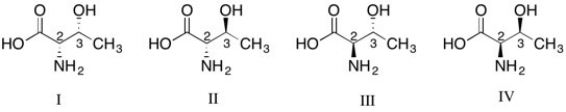
(Multiple Choice)
4.9/5  (35)
(35)
How many asymmetric carbons are present in the compound below? 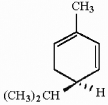
(Short Answer)
4.8/5  (28)
(28)
Is the mirror image of the following molecule an enantiomer or is it superimposable with it? 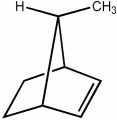
(Short Answer)
4.9/5  (34)
(34)
Is it theoretically possible to separate the pair of compounds below by distillation? Explain briefly. 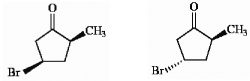
(Essay)
4.9/5  (41)
(41)
Which of the following statements is (are) true for the compound (3R, 4R)-3,4-dimethylhexane?
(Multiple Choice)
4.8/5  (26)
(26)
What term describes the structural relationship between (2R,3R,4S)-2,3,4-trichloroheptane and (2R,3R,4R)-2,3,4-trichloroheptane?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following terms best describes the pair of compounds shown: enantiomers, diastereomers, or the same compound? 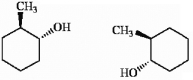
(Short Answer)
4.8/5  (28)
(28)
Which of the following statements is (are) true for the compound cis-1,2-dichlorocyclopropane?
(Multiple Choice)
4.9/5  (34)
(34)
________ are isomers which have the same bonding sequence but differ in the orientation of their atoms in space.
(Short Answer)
4.8/5  (26)
(26)
Draw the structure of (S)-3-chloro-3-methylhexane. Take particular care to indicate stereochemistry properly.
(Essay)
4.8/5  (36)
(36)
Showing 1 - 20 of 132
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)