Exam 24: Amino Acids, Peptides, and Proteins
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Which amino acid has an indole ring in its side chain?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
The solid-phase method of peptide synthesis was devised by ________.
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
B
Provide a reasonable synthesis of Gly-Val from the individual amino acids.
Free
(Essay)
4.8/5  (37)
(37)
Correct Answer:
1) Glycine + benzyl chloroformate gives N-protected Z-Gly.
2) Z-Gly is activated with ethyl chloroformate.
3) Activated Z-Gly is treated with Val to form Z-Gly-Val.
4) Benzyloxycarbonyl protecting group is removed via catalytic hydrogenation to give Gly-Val.
Which of the following reagents is used to protect the amino group of the N-terminal residue in solution-phase peptide synthesis?
(Multiple Choice)
4.8/5  (32)
(32)
A protein bonded to a sugar residue would be classified as a ________.
(Multiple Choice)
4.8/5  (31)
(31)
Provide the structure of the predominant form of L-phenylalanine present in an aqueous solution at biological pH, pH = 7.4.
(Essay)
4.9/5  (40)
(40)
Provide the structure of the major organic product which results when L-phenylalanine is treated with a large excess of PhCH2OH and gaseous HCl.
(Essay)
4.8/5  (29)
(29)
Which amino acid's side chain is positively charged at biological pH, pH = 7.4?
(Multiple Choice)
4.7/5  (35)
(35)
Which of the following structures is the neutral form of L-serine?
(Multiple Choice)
4.9/5  (45)
(45)
Provide the structure of the predominant form of lysine present in an aqueous solution at biological pH, pH = 7.4.
(Essay)
4.7/5  (32)
(32)
Even though we often write the structure of glycine as NH2CHCOOH, why does this not accurately represent the structure of the amino acid?
(Essay)
4.9/5  (31)
(31)
Provide the structure of the predominant form of L-alanine present in an aqueous solution at pH 2.
(Essay)
4.7/5  (46)
(46)
Provide the structure of the predominant form of L-valine present in an aqueous solution at biological pH, pH = 7.4.
(Essay)
4.9/5  (39)
(39)
Which of the following amino acids has its isoelectric point at the highest pH?
(Multiple Choice)
4.8/5  (28)
(28)
What is the name of the amino acid produced when propanoic acid is subjected to the following sequence of reagents: 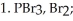
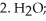
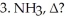
(Multiple Choice)
4.8/5  (42)
(42)
Showing 1 - 20 of 127
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)