Exam 13: Nuclear Magnetic Resonance Spectroscopy
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
Deduce the identity of the following compound from the 1H NMR data given.
C6H10O2: δ 2.19 (3H, singlet), 2.70 (2H, singlet) (ppm)
Free
(Essay)
4.8/5  (41)
(41)
Correct Answer:
CH3COCH2CH2COCH3
How many nuclear spin states are allowed for the 1H nucleus?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
B
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of CH3CH2OCH3.
Free
(Essay)
4.8/5  (34)
(34)
Correct Answer:
3 signals: (3H, triplet); (2H, quartet); (3H, singlet)
How might the proton spectrum of ultrapure dimethylamine, (CH3)2NH, differ from the spectrum of this compound to which D2O has been added?
(Essay)
4.9/5  (31)
(31)
Provide a structure that is consistent with the data below.
C9H9N
IR (cm-1): 3050, 2950, 2240, 1630
1H NMR ( d): 7.5 (2H, d), 7.1 (2H, d), 2.3 (2H, q), 0.9 (3H, t)
13C NMR (d ): 137 (s), 130, (s), 126 (d), 122 (d), 95 (s), 25 (t), 15 (q)
(Essay)
4.9/5  (42)
(42)
Predict the number of signals expected in the proton spin decoupled 13C spectrum of 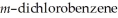 (1,3-dichlorobenzene).
(1,3-dichlorobenzene).
(Short Answer)
4.7/5  (37)
(37)
Provide a structure that is consistent with the data below.
C4H8O
IR (cm-1): 2950
1H NMR ( d): 3.2 (4H, t), 1.2 (4H, t)
13C NMR (d ): 68 (t), 27 (t)
(Essay)
4.9/5  (33)
(33)
Deduce the identity of the following compound from the 1H NMR data given.
C7H7NO3: δ 3.9 (3H, singlet), 6.9 (2H, doublet), 8.1 (2H, doublet) (ppm)
(Essay)
4.8/5  (37)
(37)
Why is Fourier transform NMR spectroscopy preferred over continuous wave as a technique for 
(Essay)
4.9/5  (42)
(42)
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of (CH3)3CCHO.
(Short Answer)
4.8/5  (32)
(32)
1H nuclei located near electronegative atoms tend to be ________ relative to 1H nuclei which are not.
(Multiple Choice)
4.9/5  (29)
(29)
What type of carbon environment does not generate a signal in the DEPT-90 spectrum and gives a positive peak in the DEPT-135 spectrum?
(Multiple Choice)
4.8/5  (33)
(33)
Predict the number of distinct quartets expected in the off-resonance decoupled  spectrum of the compound shown below.
spectrum of the compound shown below. 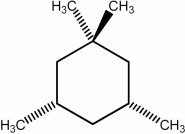
(Short Answer)
4.7/5  (29)
(29)
What is the expected multiplicity and relative ratio of the area of the peak for the protons labeled Ha? 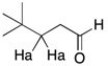
(Multiple Choice)
4.8/5  (31)
(31)
Provide a structure that is consistent with the data below.
C5H8Cl2
IR (cm-1): 2950
1H NMR ( d): 1.4 (4H, t), 1.2 (4H, t)
13C NMR (d ): 62 (s), 26 (t), 23 (t)
(Essay)
4.9/5  (35)
(35)
Provide a structure that is consistent with the data below.
C9H7Cl
IR (cm-1): 3050, 2950, 2220, 1620
1H NMR ( d): 7.8 (2H, d), 7.2 (2H, d), 2.1 (3H, s)
13C NMR (d ): 140 (s), 132, (s), 125 (d), 122 (d), 88 (s), 83 (s), 18 (q)
(Essay)
4.9/5  (32)
(32)
How might the two trimethylcyclohexane isomers shown below be most readily distinguished using NMR? 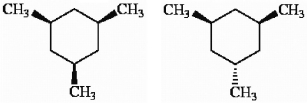
(Essay)
4.8/5  (30)
(30)
Given that compound X has a molecular formula of C6H8O2, gave two peaks in its 13C NMR spectrum (37 ppm and 208 ppm) but just one in its 1H NMR spectrum (2.5 ppm), provide a structure for compound X.
(Essay)
4.9/5  (39)
(39)
Predict the number of signals expected in the proton spin decoupled 13C NMR spectrum of the compound shown below. 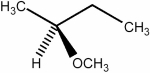
(Short Answer)
4.9/5  (40)
(40)
Deduce the identity of the following compound from the 13C NMR data given.
C9H12: δ 21.3 (quartet), 127.2 (doublet), 138.0 (singlet) (ppm)
(Short Answer)
4.9/5  (28)
(28)
Showing 1 - 20 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)