Exam 6: Alkyl Halides; Nucleophilic Substitution
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
A sample of 1-chloro-1-phenylethane with an [α]25D of -94.8° is reacted with NH3 in methanol/water solvent. The major substitution product of the reaction is 1-phenyl-1-ethylamine with an [α]25D of -8.6°. Given that optically pure (R) 1-chloro-1-phenylethane has a specific rotation of -109.0° and that optically pure (R) 1-phenyl-1-ethylamine has a specific rotation of +39.3°, which of the following statements best describes this reaction? ![A sample of 1-chloro-1-phenylethane with an [α]<sup>25</sup><sub>D</sub> of -94.8° is reacted with NH<sub>3</sub> in methanol/water solvent. The major substitution product of the reaction is 1-phenyl-1-ethylamine with an [α]<sup>25</sup><sub>D</sub> of -8.6°. Given that optically pure (R) 1-chloro-1-phenylethane has a specific rotation of -109.0° and that optically pure (R) 1-phenyl-1-ethylamine has a specific rotation of +39.3°, which of the following statements best describes this reaction?](https://storage.examlex.com/TB6199/11eab5e1_c936_76b8_9bae_7541162ce2c0_TB6199_00.jpg)
Free
(Multiple Choice)
4.7/5  (24)
(24)
Correct Answer:
A
Provide a series of synthetic steps by which (CH3)2C  CH2 could be prepared from 2-methylpropane.
CH2 could be prepared from 2-methylpropane.
Free
(Essay)
4.7/5  (42)
(42)
Correct Answer:
1) Br2, hν
2) NaOCH3, CH3OH
Predict the structure of the expected product for the following reaction. 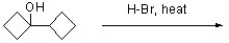
Free
(Essay)
4.9/5  (31)
(31)
Correct Answer:
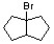
Which of the following alkyl bromides undergoes solvolysis in aqueous methanol most rapidly?
(Multiple Choice)
4.9/5  (35)
(35)
When 1-iodo-1-methylcyclohexane is treated with NaOCH2CH3, the more highly substituted alkene product predominates. When KOC(CH3)3 is used instead, the less highly substituted alkene product predominates. Offer an explanation.
(Essay)
5.0/5  (34)
(34)
Provide a structure for the major substitution and major elimination product resulting from the reaction below. 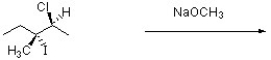
(Essay)
4.7/5  (25)
(25)
Which of the following best describes the carbon-chlorine bond of an alkyl chloride?
(Multiple Choice)
4.9/5  (34)
(34)
Do all primary iodides react with N3- at the same rate via the SN2 mechanism? Explain.
(Essay)
4.9/5  (34)
(34)
When 2,2-dimethylbutane is subjected to free-radical chlorination, ________ distinct monochlorinated products are possible and ________ of these contain asymmetric carbon atoms.
(Multiple Choice)
4.8/5  (34)
(34)
SN2 reactions take place with ________ of stereochemistry at the center undergoing substitution.
(Short Answer)
4.8/5  (30)
(30)
How many distinct alkene products results when 1-bromo-1-ethylcyclopentane is treated with NaOCH3?
(Multiple Choice)
4.8/5  (34)
(34)
The flame retardant below has been found to be an androgen agonist and may have a contribution to an increasing rate of occurrence of prostate cancer (J. Med. Chem. 2006, 7366). What is the correct term that describes the relative position of the bromides in this structure? 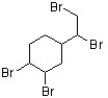
(Multiple Choice)
4.9/5  (33)
(33)
Consider the reaction of (CH3)3CO- with iodomethane. Will the reaction rate increase, decrease, or remain the same if the concentration of iodomethane is increased? Explain.
(Essay)
4.8/5  (38)
(38)
Which of the compounds below undergoes solvolysis in aqueous ethanol most rapidly?
(Multiple Choice)
4.9/5  (34)
(34)
When trans-1-iodo-4-methylcyclohexane is heated in methanol, the product mixture is primarily ________.
(Multiple Choice)
4.9/5  (37)
(37)
Draw a chair conformation of the starting substituted cyclohexane molecule with the bromine in the axial position. Using this model, predict the major elimination product of this reaction. 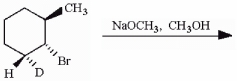
(Essay)
4.7/5  (23)
(23)
Showing 1 - 20 of 137
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)