Exam 4: The Study of Chemical Reactions
Exam 1: Structure and Bonding127 Questions
Exam 2: Acids and Bases; Functional Groups136 Questions
Exam 3: Structure and Stereochemistry of Alkanes134 Questions
Exam 4: The Study of Chemical Reactions128 Questions
Exam 5: Stereochemistry132 Questions
Exam 6: Alkyl Halides; Nucleophilic Substitution137 Questions
Exam 7: Structure and Synthesis of Alkenes; Elimination131 Questions
Exam 8: Reactions of Alkenes134 Questions
Exam 10: Structure and Synthesis of Alcohols136 Questions
Exam 11: Reactions of Alcohols125 Questions
Exam 12: Infrared Spectroscopy and Mass Spectrometry121 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy130 Questions
Exam 14: Ethers, Epoxides, and Thioethers127 Questions
Exam 15: Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy130 Questions
Exam 16: Aromatic Compounds128 Questions
Exam 17: Reactions of Aromatic Compounds129 Questions
Exam 18: Ketones and Aldehydes131 Questions
Exam 19: Amines127 Questions
Exam 20: Carboxylic Acids125 Questions
Exam 21: Carboxylic Acid Derivatives130 Questions
Exam 22: Condensations and Alpha Substitutions of Carboxyl Compounds127 Questions
Exam 23: Carbohydrates and Nucleic Acids126 Questions
Exam 24: Amino Acids, Peptides, and Proteins127 Questions
Exam 25: Lipids127 Questions
Exam 26: Synthetic Polymers128 Questions
Select questions type
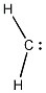
Provide the structure of the carbene that results when diazomethane (shown below) decomposes. 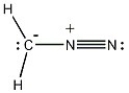
Free
(Essay)
4.8/5  (32)
(32)
Correct Answer:
Consider the reaction (CH3)3CBr + CH3CH2OH → 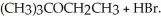 Experimentally one finds that if the concentration of (CH3)3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
Experimentally one finds that if the concentration of (CH3)3CBr is tripled, the rate of the reaction triples. One also finds that if the concentration of CH3CH2OH is doubled, the rate of the reaction is unchanged. Which of the following correctly describes the kinetics of this reaction?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
In the reaction of Cl2 with ethane and UV light, which of the following reactions would be a propagation event(s)?
I. Cl∙ + CH3-CH3 → CH3-CH2-Cl + H∙
II. Cl∙ + CH3-CH3 → CH3-H2C∙ + HCl
III. Cl∙ + CH3-H2C∙ → CH3-CH2-Cl
IV. Cl2 + CH3-H2C∙ → CH3-CH2-Cl + Cl∙
V. Cl2 + UV light → C l∙ + Cl∙
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
D
Do you expect the initiation step in the free radical chlorination of 2,2-dimethylpropane to have a positive or negative ΔS? Explain briefly.
(Essay)
4.8/5  (36)
(36)
The Arrhenius equation mathematically models which of the following statements?
(Multiple Choice)
4.8/5  (36)
(36)
Butylated hydroxytoluene, or BHT, is a potent radical scavenger that is used as a food preservative. It reacts preferentially with free radicals in food to form a new, less reactive resonance stabilized species. The reaction of BHT with a damaging free radical is shown below. Draw two additional resonance structures for the radical form of BHT where the free electron is delocalized. 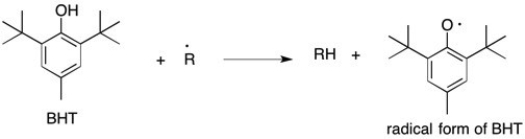
(Essay)
4.9/5  (29)
(29)
How many distinct monochlorinated products can result when cyclopentane is subjected to free radical chlorination?
(Multiple Choice)
4.8/5  (39)
(39)
When two carbenes collide, they immediately dimerize to give ________.
(Multiple Choice)
4.8/5  (37)
(37)
The rate of a reaction typically increases as the temperature increases because ________.
(Multiple Choice)
4.8/5  (40)
(40)
The bond dissociation energy is the amount of energy required to break a bond ________.
(Multiple Choice)
4.8/5  (35)
(35)
In the first propagation step of the free radical chlorination of methane, which of the following occurs?
(Multiple Choice)
4.7/5  (41)
(41)
Given a K of 0.45 at 25°C, calculate the corresponding ΔG° in kJ/mol. [R = 8.314 J/K∙ mol]
(Short Answer)
4.9/5  (34)
(34)
Write an equation to describe the initiation step in the chlorination of methane.
(Short Answer)
4.9/5  (34)
(34)
What is the hybridization of the negatively charged carbon in (CH3)3C:-?
(Short Answer)
4.9/5  (36)
(36)
Given a K of 2.2 at 25°C, calculate the corresponding ΔG° in kJ/mol. [R = 8.314 J/K∙ mol]
(Short Answer)
4.7/5  (40)
(40)
Predict the signs of ΔH° and ΔS° in the reaction of cyclohexene with H2 to form cyclohexane, shown below. 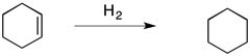
(Short Answer)
4.8/5  (31)
(31)
Given a reaction in which reactant A is converted only to product B at 25°C, what Keq results if at equilibrium 80% of A has become B?
(Short Answer)
4.8/5  (30)
(30)
Showing 1 - 20 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)