Exam 13: The Properties of Mixtures Solutions and Colloids
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Which of the following aqueous solutions should demonstrate the most non-ideal behavior?
(Multiple Choice)
5.0/5  (36)
(36)
Methane has a Henry's Law constant (k) of 9.88 × 10-2 mol/(L·atm) when dissolved in benzene at 25°C. How many grams of CH4 will dissolve in 3.00 L of benzene if the partial pressure of CH4 is 1.48 atm?
(Multiple Choice)
4.9/5  (34)
(34)
What volume of concentrated (14.7 M) phosphoric acid is needed to prepare 25.0 L of 3.0 M H3PO4?
(Multiple Choice)
4.8/5  (40)
(40)
Saccharin, one of the first non-nutritive sweeteners used in soft-drinks, is 500 times sweeter than sugar in dilute aqueous solutions. The solubility of saccharin is 1.00 gram per 290 mL of solution. What is the molarity of a saturated saccharin solution? ( 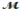 saccharin = 183.2 g/mol)
saccharin = 183.2 g/mol)
(Multiple Choice)
4.8/5  (23)
(23)
Consider the expression below, showing the terms which contribute to the heat of solution, ΔHsoln: ΔHsoln = ΔHsolute + ΔHsolvent + ΔHmix
Which of the following sets correctly shows the signs (positive or negative) of the three terms on the right hand side of the equation?
(Multiple Choice)
4.9/5  (34)
(34)
The heat of solution is the total enthalpy change when a solution is formed from the separated solute and solvent.
(True/False)
4.8/5  (50)
(50)
A 0.100 m K2SO4 solution has a freezing point of -0.43°C. What is the van't Hoff factor for this solution? Kf = 1.86°C/m
(Multiple Choice)
4.9/5  (38)
(38)
Safrole is used as a topical antiseptic. Calculate the vapor pressure of a solution prepared by dissolving 0.75 mol of safrole in 950 g of ethanol ( 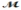 = 46.07 g/mol). P°ethanol = 50.0 torr at 25°C.
= 46.07 g/mol). P°ethanol = 50.0 torr at 25°C.
(Multiple Choice)
4.9/5  (28)
(28)
The chemist, Anna Lytic, must prepare 1.00 kg of 15.0% (w/w) acetic acid using a stock solution which is 36.0% (w/w) acetic acid (d = 1.045 g/mL). Which of the following combinations will give her the solution she wants?
(Multiple Choice)
4.7/5  (36)
(36)
Raoult's Law relates the vapor pressure of the solvent above the solution to its mole fraction in the solution. Which of the following is an accurate statement?
(Multiple Choice)
4.9/5  (41)
(41)
The concentration of iodine in sea water is 60. parts per billion by mass. If one assumes that the iodine exists in the form of iodide anions, what is the molarity of iodide in sea water? (The density of sea water is 1.025 g/mL.)
(Multiple Choice)
4.9/5  (40)
(40)
Copper(II) bromide is used as a wood preservative. What mass of CuBr2 is needed to prepare 750.0 mL of a 1.25 M solution?
(Multiple Choice)
4.8/5  (30)
(30)
Two aqueous solutions are prepared: 2.0 m Cu(NO3)2 and 2.0 m NaBr. Which of the following statements is true?
(Multiple Choice)
5.0/5  (37)
(37)
In general, water is a good solvent for both polar and nonpolar compounds.
(True/False)
4.8/5  (40)
(40)
Cinnamaldehyde ( 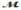 = 132.15 g/mol) is used as a flavoring agent. What mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 82.7°C? Kb = 1.22°C/m, boiling point of pure ethanol = 78.5°C
= 132.15 g/mol) is used as a flavoring agent. What mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 82.7°C? Kb = 1.22°C/m, boiling point of pure ethanol = 78.5°C
(Multiple Choice)
4.7/5  (39)
(39)
Aqueous ammonia is commercially available in a solution that is 28% (w/w) ammonia. What is the mole fraction of ammonia in such a solution?
(Multiple Choice)
4.8/5  (30)
(30)
Calculate the molarity of a solution prepared by diluting 1.85 L of 6.5 M KOH to 11.0 L.
(Multiple Choice)
4.8/5  (38)
(38)
The mole fraction of potassium nitrate in an aqueous solution is 0.0194. The solution's density is 1.0627 g/mL. Calculate the molarity of the solution.
(Multiple Choice)
4.8/5  (40)
(40)
Showing 41 - 60 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)