Exam 13: The Properties of Mixtures Solutions and Colloids
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Raoult's Law relates the vapor pressure of a solvent above a solution to the mole fraction of the solvent and the vapor pressure of the pure solvent.
(True/False)
4.8/5  (37)
(37)
Calculate the vapor pressure of a solution prepared by dissolving 0.500 mol of a non-volatile solute in 275 g of hexane ( 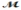 = 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
= 86.18 g/mol) at 49.6°C. P°hexane = 400.0 torr at 49.6°C.
(Multiple Choice)
4.9/5  (25)
(25)
Benzaldehyde ( 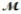 = 106.1 g/mol), also known as oil of almonds, is used in the manufacture of dyes and perfumes and in flavorings. What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m, freezing point of pure ethanol = -117.3°C.
= 106.1 g/mol), also known as oil of almonds, is used in the manufacture of dyes and perfumes and in flavorings. What would be the freezing point of a solution prepared by dissolving 75.00 g of benzaldehyde in 850.0 g of ethanol? Kf = 1.99°C/m, freezing point of pure ethanol = -117.3°C.
(Multiple Choice)
4.8/5  (32)
(32)
Potassium fluoride is used for frosting glass. Calculate the molarity of a solution prepared by dissolving 78.6 g of KF in enough water to produce 225 mL of solution.
(Multiple Choice)
4.7/5  (45)
(45)
The solubility of gases in water increases with increase in the pressure of the gas.
(True/False)
4.8/5  (41)
(41)
A solution of sucrose (sugar) in water is in equilibrium with solid sucrose. If more solid sucrose is now added, with stirring,
(Multiple Choice)
4.8/5  (31)
(31)
From the following list of aqueous solutions and water, select the one with the highest boiling point.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following pairs of ions is arranged so that the ion with the larger (i.e., more negative) heat of hydration is listed first?
(Multiple Choice)
4.7/5  (36)
(36)
Children under the age of 6 with more than 0.10 ppm of lead in their blood can suffer a reduction in I.Q. or have behavior problems. What is the molality of a solution which contains 0.10 ppm of lead?
(Multiple Choice)
4.8/5  (41)
(41)
Isoamyl salicylate ( 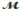 = 208.25 g/mol) has a pleasant aroma and is used in perfumes and soaps. Which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/mL)?
= 208.25 g/mol) has a pleasant aroma and is used in perfumes and soaps. Which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/mL)?
(Multiple Choice)
4.8/5  (41)
(41)
Based only on the relative lattice energies of the compounds below, which one would be expected to have the lowest solubility in water?
(Multiple Choice)
4.8/5  (35)
(35)
Human blood has a molar concentration of solutes of 0.30 M. What is the osmotic pressure of blood at 25°C?
(Multiple Choice)
4.9/5  (45)
(45)
Diethyl ether has a vapor pressure of 400.0 torr at 18°C. When a sample of benzoic acid is dissolved in ether, the vapor pressure of the solution is 342 torr. What is the mole fraction of benzoic acid in the solution?
(Multiple Choice)
4.8/5  (48)
(48)
Gases with high boiling points tend to be more soluble in water than ones with low boiling points.
(True/False)
4.8/5  (37)
(37)
A solution of ethanol (C2H6O) in water is sometimes used as a disinfectant. 1.00 L of this solution contains 553 g of ethanol and 335 g of water. What is the molality of the ethanol in this solution?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 61 - 80 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)