Exam 16: Aqueous Ionic Equilibrium
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
If the pKa of HCOOH is 3.74 and the pH of an HCOOH/HCOONa solution is 3.11, which of the following is TRUE?
(Multiple Choice)
4.7/5  (40)
(40)
Determine the molar solubility of BaF2 in a solution containing 0.0750 mol L-1 LiF. Ksp (BaF2) = 1.7 × 10-6.
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following is the correct equation relating Q to Ksp for an unsaturated solution?
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following acids (listed with pKa values) and its conjugate would be the best choice to make a buffer with a pH of 2.12?
(Multiple Choice)
4.9/5  (34)
(34)
A 25.0 mL sample of 0.150 mol L-1 formic acid is titrated with a 0.150 mol L-1 NaOH solution. What is the pH at the equivalence point? The 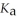 of formic acid is 1.8 × 10-4.
of formic acid is 1.8 × 10-4.
(Multiple Choice)
4.9/5  (39)
(39)
A 100.0 mL sample of 0.20 mol L-1 HF is titrated with 0.10 mol L-1 KOH. Determine the pH of the solution before the addition of any KOH. The Ka of HF is 3.5 × 10-4.
(Multiple Choice)
4.9/5  (38)
(38)
If the pKa of HCOOH is 3.74 and the pH of an HCOOH/HCOONa buffer solution is 3.74, which of the following is TRUE?
(Multiple Choice)
4.7/5  (44)
(44)
What is the pH of a solution prepared by mixing 25.00 mL of 0.10 mol L-1 CH3COOH with 25.00 mL of 0.0 10 mol L-1 CH3COONa? Assume that the volume of the solutions are additive and that Ka = 1.8 × 10-5 for CH3COOH.
(Multiple Choice)
4.7/5  (32)
(32)
When titrating a weak monoprotic acid with NaOH at 25 °C, the ________.
(Multiple Choice)
4.9/5  (29)
(29)
A sample contains Ba3(PO4)2, CdS, AgCl, NH4Cl, and ZnS. Identify the precipitate formed upon the addition of 6 mol L-1 HCl.
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following compounds' solubility will not be affected by a low pH in solution?
(Multiple Choice)
4.8/5  (42)
(42)
What is the [CH3COO-]/[CH3COOH] ratio necessary to make a buffer solution with a pH of 4. 34? Ka = 1.8 × 10-5 for CH3COOH.
(Multiple Choice)
4.8/5  (35)
(35)
Determine the molar solubility of BaF2 in pure water. Ksp for BaF2 = 2.45 × 10-5.
(Multiple Choice)
4.8/5  (44)
(44)
A 100.0 mL sample of 0.10 mol L-1 NH3 is titrated with 0.10 mol L-1 HNO3. Determine the pH of the solution after the addition of 100.0 mL of HNO3. The Kb of NH3 is 1.8 × 10-5.
(Multiple Choice)
4.9/5  (34)
(34)
A 1.00 L buffer solution is 0.150 mol L-1 in C6H5COOH and 0.250 mol L-1 in C6H5COOLi. Calculate the pH of the solution after the addition of 100.0 mL of 1.00 mol L-1 HCl. The Ka for HC7H5O2 is 6.5 × 10-5.
(Multiple Choice)
4.9/5  (34)
(34)
What is the pH of the resulting solution if 45 mL of 0.432 mol L-1 methylamine, CH3NH2, is added to 15 mL of 0.234 mol L-1 HCl? Assume that the volumes of the solutions are additive. Ka = 2.70 × 10-11 for CH3NH3+.
(Multiple Choice)
4.9/5  (40)
(40)
A solution containing AgNO3 is mixed with a solution of NaCl to form a solution that is 0.10 mol L-1 in AgNO3 and 0.075 mol L-1 in NaCl. What will happen once these solutions are mixed? Ksp (AgCl) = 1.77 × 10-10.
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the mole ratio of C6H5COONa to C6H5COOH required to make a buffer with pH of 4.68. Ka of C6H5COOH is 6.3 × 10-5.
(Multiple Choice)
4.9/5  (27)
(27)
What is the approximate pH at the equivalence point of a weak acid-strong base titration if 25 mL of aqueous formic acid (HCOOH) requires 29.80 mL of 0. 3567 mol L-1 NaOH? Ka =1.8 × 10-4  for formic acid.
for formic acid.
(Multiple Choice)
4.9/5  (32)
(32)
What volume of 5.00 × 10-3 mol L-1 HNO3 is needed to titrate 80.00 mL of 5.00 × 10-3 mol L-1 Ca(OH)2 to the equivalence point?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 21 - 40 of 173
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)