Exam 16: Aqueous Ionic Equilibrium
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
A 25.0 mL sample of 0.150 mol L-1 butanoic acid is titrated with a 0.150 mol L-1 NaOH solution. What is the pH after 13.3 mL of base is added? The 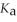 of butanoic acid is 1.5 × 10-5.
of butanoic acid is 1.5 × 10-5.
(Multiple Choice)
4.9/5  (36)
(36)
Identify the expression for the solubility product constant for PbCl2.
(Multiple Choice)
4.7/5  (40)
(40)
Describe the information necessary (and why) to choose a good indicator for a weak acid-strong base titration.
(Essay)
4.8/5  (35)
(35)
Which of the following is the correct equation relating Q to Ksp for a supersaturated solution?
(Multiple Choice)
4.9/5  (41)
(41)
For a strong acid titrated with a strong base, is the equivalence point acidic, basic, or neutral?
(Short Answer)
4.9/5  (29)
(29)
A 100.0 mL sample of 0.18 mol L-1 HClO4 is titrated with 0.27 mol L-1 NaOH. Determine the pH of the solution after the addition of 66.67 mL of NaOH (this is the equivalence point).
(Multiple Choice)
4.8/5  (37)
(37)
A 1.00 L buffer solution is 0.250 mol L-1 in HF and 0.250 mol L-1 in LiF. Calculate the pH of the solution after the addition of 0.150 moles of solid LiOH. Assume no volume change upon the addition of the base. The Ka for HF is 3.5 × 10-4.
(Multiple Choice)
4.8/5  (48)
(48)
What is the pH at the equivalence point of a weak base-strong acid titration if 20.00 mL of NaOCl requires 28.30 mL of 0. 50 mol L-1 HCl for complete neutralization? Ka = 3.0 × 10-8 for HOCl.
(Multiple Choice)
4.9/5  (34)
(34)
The molar solubility of Ag2S is 1.26 × 10-16 mol L-1 in pure water. Calculate the Ksp for Ag2S.
(Multiple Choice)
4.8/5  (43)
(43)
A 25.0 mL sample of 0.150 mol L-1 acetic acid is titrated with a 0.150 mol L-1 NaOH solution. What is the pH after 26.0 mL of base is added? The 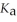 of acetic acid is 1.8 × 10-5.
of acetic acid is 1.8 × 10-5.
(Multiple Choice)
4.8/5  (42)
(42)
A 100.0 mL sample of 0.10 mol L-1 NH3 is titrated with 0.10 mol L-1 HNO3. Determine the pH of the solution after the addition of 200.0 mL of HNO3. The Kb of NH3 is 1.8 × 10-5.
(Multiple Choice)
4.9/5  (32)
(32)
You wish to prepare a buffer containing CH3COOH with a pH of 4.24. If the pKa of acetic acid is 4.74, what ratio of [CH3COO⁻]/[CH3COOH] must you use?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following acids (listed with Ka values) and their conjugate base would be the best choice to make a buffer with a pH of 2.34?
(Multiple Choice)
4.8/5  (45)
(45)
A solution contains 0.021 mol L-1 Cl⁻ and 0.017 mol L-1 I⁻. A solution containing copper(I) ions is added to selectively precipitate one of the ions. At what concentration of copper(I) ion will a precipitate begin to form? What is the identity of the precipitate? Ksp(CuCl) = 1.0 × 10-6, Ksp(CuI) = 5.1 × 10-12.
(Multiple Choice)
4.9/5  (39)
(39)
A 1.0 L buffer solution is 0.250 mol L-1 CH3COOH and 0.050 mol L-1 CH3COOLi. Which of the following actions will exceed the capacity of the buffer?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following acids (listed with Ka values) and their conjugate base would be the best choice to make a buffer with a pH of 8.10?
(Multiple Choice)
4.9/5  (31)
(31)
A solution contains 0.036 mol L-1 Cu2+ and 0.044 mol L-1 Fe2+. A solution containing sulfide ions is added to selectively precipitate one of the metal ions from solution. At what concentration of sulfide ion will a precipitate begin to form? What is the identity of the precipitate? Ksp(CuS) = 1.3 × 10-36, Ksp(FeS) = 6.3 × 10-18.
(Multiple Choice)
4.7/5  (34)
(34)
Showing 41 - 60 of 173
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)