Exam 8: Basic Concepts of Chemical Bonding
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Calculate the bond energy of C- F given that the heat of atomization of CHFClBr is 1502 kJ/mol, and that the bond energies of C- H, C- Br, and C- Cl are 413, 276, and 328 kJ/mol, respectively.
(Essay)
4.8/5  (36)
(36)
For the questions that follow, consider the BEST Lewis structures of the following ox_yanions: 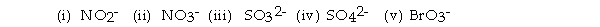 -There can be four equivalent best resonance structures of .
-There can be four equivalent best resonance structures of .
(Multiple Choice)
4.8/5  (39)
(39)
What is the maximum number of double bonds that a carbon atom can form?
(Multiple Choice)
4.8/5  (32)
(32)
For a given arrangement of ions, the lattice energy increases as ionic radius and as ionic charge .
(Multiple Choice)
4.9/5  (38)
(38)
How many single covalent bonds must a silicon atom form to have a complete octet in its valence shell?
(Multiple Choice)
4.7/5  (20)
(20)
In which of the molecules below is the carbon- carbon distance the shortest?
(Multiple Choice)
4.8/5  (30)
(30)
The ability of an atom in a molecule to attract electrons is best quantified by the .
(Multiple Choice)
4.9/5  (35)
(35)
There are unpaired electrons in the Lewis symbol for an oxygen atom.
(Multiple Choice)
4.8/5  (42)
(42)
How many equivalent resonance structures can be drawn for the molecule of SO3 without having to violate the octet rule on the sulfur atom?
(Multiple Choice)
4.8/5  (35)
(35)
Electronegativity _ from left to right within a period and _ from top to bottom within a group.
(Multiple Choice)
4.8/5  (29)
(29)
How many equivalent resonance forms can be drawn for CO32- - (carbon is the central atom)?
(Multiple Choice)
4.9/5  (33)
(33)
In a reaction, if the bonds in the reactants are stronger than the bonds in the product, the reaction is .
(Short Answer)
4.8/5  (34)
(34)
A valid Lewis structure of _ _ cannot be drawn without violating the octet rule.
(Multiple Choice)
4.7/5  (30)
(30)
Showing 61 - 80 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)