Exam 8: Basic Concepts of Chemical Bonding
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Which of the following has the bonds correctly arranged in order of increasing polarity?
(Multiple Choice)
4.8/5  (21)
(21)
The principal quantum number of the electrons that are lost when tungsten forms a caton is
(Multiple Choice)
4.8/5  (37)
(37)
There are paired and unpaired electrons in the Lewis symbol for a phosphorus atom.
(Multiple Choice)
4.7/5  (27)
(27)
Using the table of bond dissociation energies, the OH for the following gas- phase reaction is
KJ)
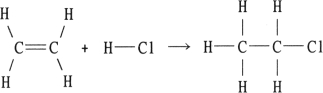
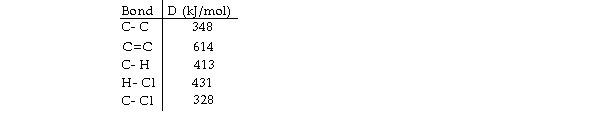
(Multiple Choice)
4.8/5  (30)
(30)
Which one of the following species has the electron configuration [Ar]3d4?
(Multiple Choice)
4.7/5  (39)
(39)
The electron configuration of the phosph?ide ion (P3- ) is .
(Multiple Choice)
4.7/5  (32)
(32)
Which of the elements below has the largest electronegativity?
(Multiple Choice)
4.9/5  (37)
(37)
Why don't we draw double bonds between the Be atom and the Cl atoms in BeCl2?
(Multiple Choice)
4.7/5  (41)
(41)
In the resonance form of ozone shown below, the formal charge on the central oxygen atom is
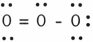
(Multiple Choice)
4.7/5  (32)
(32)
Write the balanced chemical equation for the reaction for which OH°rxn is the lattice energy for potassium bromide.
(Essay)
4.7/5  (34)
(34)
Electron affinity is a measure of how strongly an atom can attract additional electrons.
(True/False)
4.8/5  (37)
(37)
A nonpolar bond will form between two _ _ atoms of electronegativity.
(Multiple Choice)
4.8/5  (32)
(32)
How many different types of resonance structures can be drawn for the ion SO32- where all atoms satisfy the octet rule ?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 81 - 100 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)