Exam 8: Basic Concepts of Chemical Bonding
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Which of the following would have to lose two electrons in order to achieve a noble gas electron configuration ? 
(Multiple Choice)
4.8/5  (38)
(38)
In the Lewis symbol for a fluorine atom, there are paired and unpaired electrons.
(Multiple Choice)
4.8/5  (34)
(34)
A valid Lewis structure of _ _ cannot be drawn without violating the octet rule.
(Multiple Choice)
4.8/5  (35)
(35)
Of the following, cannot accommodate more than an octet of electrons.
(Multiple Choice)
4.7/5  (41)
(41)
Using the table of bond dissociation energies, the OH for the following gas- phase reaction is
KJ)
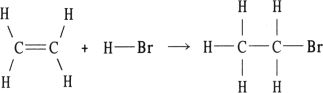

(Multiple Choice)
4.9/5  (42)
(42)
Of the possible bonds between carbon atoms (single, double, and triple), _.
(Multiple Choice)
4.9/5  (38)
(38)
As the number of covalent bonds between two atoms increases, the distance between the atoms
And the strength of the bond between them _ _.
(Multiple Choice)
4.8/5  (44)
(44)
As electronegativity difference increases, bond length will decrease.
(True/False)
4.8/5  (32)
(32)
The electron configuration that corresponds to the Lewis symbol, :
(Short Answer)
4.8/5  (38)
(38)
The formal charge on sulfur in SO42- is , where the Lewis structure of the ion is:
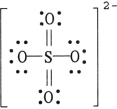
(Multiple Choice)
4.7/5  (31)
(31)
The type of compound that is most likely to contain a covalent bond is _.
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following would have to gain two electrons in order to achieve a noble gas electron configuration _ ?
O Sr Na Se Br
(Multiple Choice)
4.8/5  (31)
(31)
Using the table of average bond energies below, the OH for the reaction is kJ. 
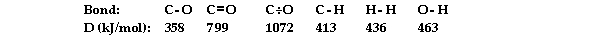
(Multiple Choice)
4.9/5  (30)
(30)
The bond length in an HI molecule is 1.61 Å and the measured dipole moment is 0.44 D. What is the magnitude (in units of e) of the negative charge on I in HI?
(1 debye = 3.34 × 10- 34 coulomb- meters; ; e=1.6 × 10- 19 coulombs)
(Multiple Choice)
4.9/5  (31)
(31)
Showing 21 - 40 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)