Exam 17: Additional Aspects of Equilibria
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Consider the following table of Ksp values. 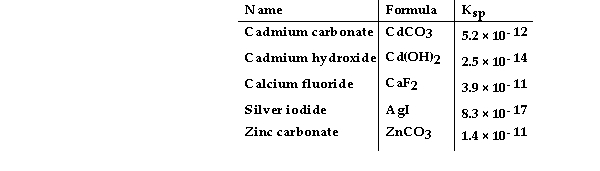 -Which compound listed below has the smallest molar solubility in water?
-Which compound listed below has the smallest molar solubility in water?
(Multiple Choice)
4.7/5  (33)
(33)
200.0 ml of a solution containing 0.5000 moles of acetic acid per liter is added to 200.0 ml of 0.5000 M NaOH. What is the final pH? The Ka of acetic acid is 1.770 × 10- 5.
(Short Answer)
4.9/5  (36)
(36)
What is the pH of a buffer solution that is 0.211 M in lactic acid and 0.111 M in sodium lactate? The Ka of lactic acid is 1.37 × 10- 4.
(Multiple Choice)
4.8/5  (36)
(36)
What is the molar solubility of magnesium carbonate ( MgCO3 ) in water? The solubility- product constant for MgCO3 is 3.5 × 10- 8 at 25°C.
(Multiple Choice)
4.9/5  (40)
(40)
How many milliliters of 0.0850 M NaOH are required to titrate 25.0 mL of 0.0720 M HBr to the equivalence point?
(Multiple Choice)
4.7/5  (39)
(39)
Suppose you have just added 200.0 ml of a solution containing 0.5000 moles of acetic acid per liter to 100.0 ml of 0.5000 M NaOH. What is the final pH? The Ka of acetic acid is 1.770× 10- 5.
(Short Answer)
4.9/5  (41)
(41)
A solution is prepared by dissolving 0.23 mol of hydrazoic acid and 0.27 mol of sodium azide in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly. The pH does not increase drastically because the NaOH reacts with the present in the buffer solution. The Ka of hydrazoic acid is 1.9 × 10- 5.
(Multiple Choice)
4.7/5  (39)
(39)
In which of the following aqueous solutions would you expect AgCl to have the lowest solubility?
(Multiple Choice)
4.8/5  (23)
(23)
Showing 81 - 88 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)