Exam 24: Organic Compounds
Explain the characteristics of arenes and the reactions they undergo.
Most arenes that contain a single six-membered ring are volatile liquids, such as benzene and the xylenes, although some arenes with substituents on the ring are solids at room temperature. Although arenes are usually drawn with three C=C bonds, benzene is about 150 kJ/mol more stable than would be expected if it contained three double bonds. This increased stability is due to the delocalization of the π electron density over all the atoms of the ring. Compared with alkenes, arenes are poor nucleophiles. They do not undergo addition reactions like alkenes instead, they undergo a variety of electrophilic aromatic substitution reactions that involve the replacement of -H on the arene by a group -E, such as -NO2, -SO3H, a halogen, or an alkyl group, in a two-step process. The first step involves addition of the electrophile to the π system of benzene, forming a carbocation. In the second step, a proton is lost from the adjacent carbon on the ring.
Which of the following carbohydrates is a monosaccharide?
B
What are the three stages in radical chain reactions?
Many important organic reactions involve radicals, such as the combustion of fuels. Probably the best known is reacting a saturated hydrocarbon, such as ethane, with a halogen, such as Br2. The overall reaction is as follows:
CH3CH3 + Br2 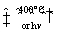 CH3CH2Br + HBr
CH3CH2Br + HBr
Radical chain reactions occur in three stages: initiation, propagation, and termination. At high temperature or in the presence of light, the relatively weak Br-Br bond is broken in an initiation step that produces an appreciable number of Br atoms (Br∙). During propagation, a bromine atom attacks ethane, producing a radical, which then reacts with another bromine molecule to produce ethyl bromide. The sum of the two propagation steps corresponds to the balanced chemical equation for the overall reaction. There are three possible termination steps: the combination of
(1) two bromine atoms,
(2) two ethyl radicals, or
(3) an ethyl and a bromine radical.
Delocalization of π bonding over three atoms makes carboxylic acids and their derivatives more susceptible to nucleophilic attack than aldehydes and ketones with their single π bond.
A reaction in which the components of a species A-B are added to adjacent atoms across a carbon-carbon multiple bond is called a(n) _____ reaction.
Which of the following methods is used to prepare aldehydes?
Optical isomers have identical physical properties, although their chemical properties may differ in asymmetric environments.
_____ are esters produced by the nucleophilic attack of an alcohol on the carbonyl carbon of a long-chain carboxylic acid.
Which of the following methods is used to prepare alcohols?
The structural units that chemists use to classify organic compounds, and to predict their reactivities under a given set of conditions are called _____.
_____ are molecules whose structures are mirror images but cannot be superimposed on one another in any orientation.
A(n) _____ is a highly reactive species that has an unpaired valence electron.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)