Exam 8: Ionic Versus Covalent Bonding
What is the formal charge of boron in BH3?
A
If the bonds in the products are stronger than those in the reactants, the reaction is exothermic.
True
Explain the relationship between lattice energies and physical properties.
The melting point is the temperature at which the individual ions have enough kinetic energy to overcome the attractive forces that hold them in place. At the melting point, the ions can move freely, and the substance becomes a liquid. Thus, melting points vary with lattice energies for ionic substances that have similar structures.
The Hardness of ionic materials, that is, their resistance to scratching or abrasion, is also related to their lattice energies. Hardness is directly related to how tightly the ions are held together electrostatically, which, is also reflected in the lattice energy. In addition to determining melting point and Hardness, lattice energies affect the solubilities of ionic substances in water. In general, the higher the lattice energy, the less soluble a compound is in water.
Which of the following compounds has the highest lattice energy?
_____ molecules are those which have less than an octet of electrons around one atom.
The number of dots in the Lewis dot symbol is the same as the number of valence electrons.
Covalent bonding signifies that positively and negatively charged ions are held together by electrostatic forces.
Given: The Lewis structure of BeCl2. 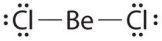 The structure shown above is an exception to the octet rule because the arrangement:
The structure shown above is an exception to the octet rule because the arrangement:
Energy of the electrostatic attraction (E), a measure of the force's strength, is inversely proportional to the _____between the charged particles, where each ion's charge is represented by the symbol Q.
The _____ is a balance between the repulsive interactions between electrons on adjacent ions and the attractive interactions between ions with opposite charges.
A system consisting of separate ion pairs is more stable than an ionic lattice.
In the reaction (CH3)2O + BF3 → (CH3)2O:BF3, the Lewis acid is _____.
Explain the significance of the energy of the electrostatic attraction.
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)