Exam 13: Kinetics: Mechanisms and Rates of Reactions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
For a large number of reactions in organic chemistry, an increase in temperature 10°C over room temperature will double the rate. What activation energy does this correspond to?
(Short Answer)
4.9/5  (37)
(37)
The reaction of NO2 with CO to give CO2 and NO can proceed through different mechanisms. What first step would be consistent with the following rate law?Rate = k[NO2][CO]
(Short Answer)
4.9/5  (33)
(33)
Which of the following are bimolecular processes?
I. 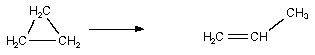 II. NO ONNO
III. H2C = CHCH3 + H2SO4 CH3CH(OSO2(OH))CH3
IV.
II. NO ONNO
III. H2C = CHCH3 + H2SO4 CH3CH(OSO2(OH))CH3
IV.  V. H2 2 H•
V. H2 2 H•
(Multiple Choice)
4.8/5  (33)
(33)
Consider the aqueous phase reaction between the dichromate anion and iron (II) cations:14 H3O+(aq) + Cr2O72- + 6Fe2+(aq) 2Cr3+(aq) + 21H2OWhat is the rate of increase of Cr3+ concentration expressed in terms of changing H3O+ concentration?
(Multiple Choice)
4.9/5  (27)
(27)
The reaction of NO with O2 to give oxygen is known to follow a third order rate law(rate = k[NO]2[O2]). Two possible mechanisms are shown below: ![The reaction of NO with O<sub>2</sub> to give oxygen is known to follow a third order rate law(rate = k[NO]<sup>2</sup>[O<sub>2</sub>]). Two possible mechanisms are shown below: Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?](https://storage.examlex.com/TB9687/11ee726d_d441_af11_827e_a1cabc6566d2_TB9687_00.jpg) Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?
Which of these two mechanisms is a more acceptable mechanism, based on the criteria given above?
(Multiple Choice)
4.9/5  (40)
(40)
It is determined that the charcoal in a fire pit used as an ancient hearth has lost about 42.3% of the initial 14C. How old was the fire pit if 14C has a half life of 5730 years?
(Multiple Choice)
4.7/5  (42)
(42)
At moderate temperatures, the rate law for the reaction of NO2 and CO to give CO2 and NO follows the rate law shown below:rate = k [NO2]2 ![At moderate temperatures, the rate law for the reaction of NO<sub>2</sub> and CO to give CO<sub>2</sub> and NO follows the rate law shown below:rate = k [NO<sub>2</sub>]<sup>2</sup> In which flask will the reaction be faster and how much faster?](https://storage.examlex.com/TB9687/11ee726d_d443_0eae_827e_b9db2beea02d_TB9687_00.jpg) In which flask will the reaction be faster and how much faster?
In which flask will the reaction be faster and how much faster?
(Short Answer)
4.7/5  (26)
(26)
The rate law for the reaction of NO with O2 to give NO2 is shown below:rate = k [NO]2[O2]
a) If all other conditions are kept constant, what will be the effect on the rate if the concentration of NO is doubled?
b) If all other conditions are kept constant, what will be the effect on the rate if the concentration of O2 is doubled?
(Short Answer)
4.8/5  (40)
(40)
The reaction of NO2 with CO to give CO2 and NO can proceed through different mechanisms. What rate law would be consistent for the following first step?
2 NO2 NO + NO3
(Short Answer)
4.8/5  (35)
(35)
Cyclohexane is manufactured from the reaction of benzene with hydrogen:
C6H6(g) + 3 H2(g) C6H12(g)
If the initial concentration of hydrogen was 1.5 M and 5 minutes later the hydrogen concentration is 0.34 M, what is the average rate of appearance of cyclohexane?
(Multiple Choice)
4.9/5  (30)
(30)
Sucrose, cane sugar, reacts with water in acid solution to give glucose and fructose, which have the same chemical formula.
C12H22O11 (aq) + H2O (l) 2 C6H12O6 (aq)
The following data were obtained at room temperature for sucrose: 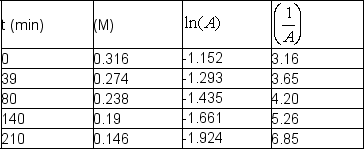 Use graphical means to determine the order of the reaction and write the rate law with the numerical value of the rate constant with time units of seconds.
Use graphical means to determine the order of the reaction and write the rate law with the numerical value of the rate constant with time units of seconds.
(Short Answer)
4.8/5  (31)
(31)
Which of the following does NOT occur during a reaction facilitated by a heterogeneous catalyst?
(Multiple Choice)
4.7/5  (34)
(34)
The reaction A + 2B products was found to have the rate law;
rate = k[A] [B]2. While holding the concentration of A constant, the concentration of B was increased from 0.010M to 0.030M. Predict by what factor the rate of reaction will increase.
(Multiple Choice)
4.8/5  (39)
(39)
Nitrogen dioxide, NO2 will react with carbon monoxide, CO, to form nitric oxide, NO, and carbon dioxide, CO2. A proposed mechanism is:
2NO2 NO3 + NO
NO3 + CO NO2 + CO2
Experiments indicate that the rate of the reaction is independent of the CO concentration. Identify the rate determining step and derive the rate law consistent with the mechanism and experimental observation.
(Short Answer)
4.9/5  (31)
(31)
A 1.66 x 10-4 mole sample of 239Pu undergoes 9 x 107 decays per second obeying first-order kinetics. How many decays per second would be expected from a 5.46 x 10-1 mole sample?
(Multiple Choice)
4.8/5  (31)
(31)
Hydrogen and iodine react to form HI. One possible mechanism is shown below:I2(g)  2 I(g)
H2(g) + 2 I(g) 2 HI(g)
Consider the following statements in light of this mechanism:
I. The rate law overall is second order.
II. The iodine atom is an intermediate.
III. The first step is the rate determining step.
IV. The second step is the fast step.
V. The second step is rate determining.
Which of the above statements are true?
2 I(g)
H2(g) + 2 I(g) 2 HI(g)
Consider the following statements in light of this mechanism:
I. The rate law overall is second order.
II. The iodine atom is an intermediate.
III. The first step is the rate determining step.
IV. The second step is the fast step.
V. The second step is rate determining.
Which of the above statements are true?
(Multiple Choice)
4.7/5  (45)
(45)
A particular first-order reaction is characterized by activation energy of 50 kJ/mole. At what temperature would the rate of the reaction be 10 times that at 298oK?
(Short Answer)
4.8/5  (47)
(47)
For the following reaction A + B C + D, the rate law is determined to be Rate = k[A]2a) Of the five proposed mechanisms shown below, which is consistent with the experimentally determined rate law?
1. 2 A Z (slow)2 B + Z 2 C + 2 D (fast)
2. A + B C + D (slow)
3. 2 B N (slow)2 A + N 2 C + 2 D (fast)
4. A X (slow)B + X C + D (fast)
5. B M (slow)A + M C + D (fast)b)
Are there any intermediates in the mechanism you chose and if so what?
(Short Answer)
4.8/5  (45)
(45)
Showing 41 - 60 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)