Exam 13: Kinetics: Mechanisms and Rates of Reactions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
What are the units of a rate constant for a reaction that has an overall order of 3?
(Short Answer)
4.8/5  (37)
(37)
Write the overall equation of reaction for the following mechanism and identify the reaction intermediates:
2 NO2 NO3 + NO
NO3 + CO CO2 + NO2
(Essay)
4.9/5  (43)
(43)
Consider the following energy-reaction coordinate diagram. 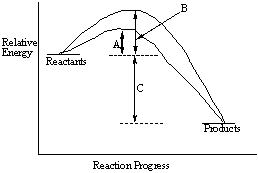 Give the names for the quantities indicated by A, B and C.
Give the names for the quantities indicated by A, B and C.
(Essay)
4.9/5  (38)
(38)
The following concentration vs. time data were collected for the reaction:
A + 2B C 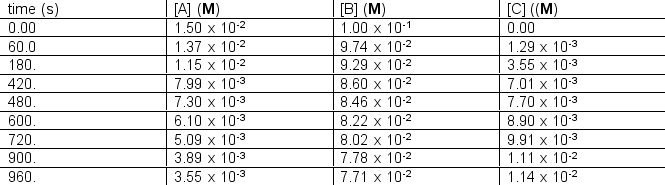 Calculate
Calculate 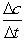 for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
(Essay)
4.7/5  (34)
(34)
Write the overall equation of reaction for the following mechanism and identify the reaction intermediates:
Cl2 2 Cl•
Cl• + CO COCl
COCl + Cl2 COCl2 + Cl•
2 Cl• Cl2
(Essay)
4.8/5  (36)
(36)
Consider the following three molecular pictures that represent the relative numbers of the two reactants involved in one step of the depletion of stratospheric ozone by chlorine atoms: 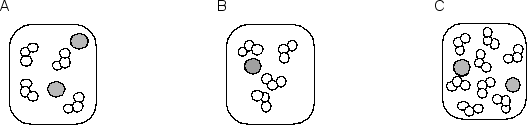 The equation for the elementary reaction and a molecular picture of the reaction process are shown below:Cl• + O3 ClO + O2
The equation for the elementary reaction and a molecular picture of the reaction process are shown below:Cl• + O3 ClO + O2  If the three samples represented by A, B and C are at the same temperature, what are the rates of reaction of B and C compared to that of A?
If the three samples represented by A, B and C are at the same temperature, what are the rates of reaction of B and C compared to that of A?
(Essay)
4.7/5  (41)
(41)
Rate data were collected for the following reaction at a constant temperature.2ClO2(aq) + 2 OH-1(aq) ClO3-1(aq) + ClO2-1(aq) + H2O(l) 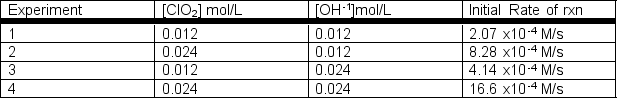 a) Determine the rate law for this reaction.b) Determine the rate constant with appropriate units.
a) Determine the rate law for this reaction.b) Determine the rate constant with appropriate units.
(Short Answer)
4.8/5  (43)
(43)
The following mechanism has been suggested for the decomposition of ozone, O3.O3(g)  O2(g) + O(g) (fast equilibrium)
O(g) + O3(g) 2 O2(g) (slow)
Consider the following statements in light of this mechanism:
I. The rate law is second order in O3.
II. The rate does not depend on the concentration of O2.
III. The reaction slows with increased O2 concentration.
IV. The rate law is second order.
V. Substances reacting with O atoms will speed up the reaction.Which of the above statements are true?
O2(g) + O(g) (fast equilibrium)
O(g) + O3(g) 2 O2(g) (slow)
Consider the following statements in light of this mechanism:
I. The rate law is second order in O3.
II. The rate does not depend on the concentration of O2.
III. The reaction slows with increased O2 concentration.
IV. The rate law is second order.
V. Substances reacting with O atoms will speed up the reaction.Which of the above statements are true?
(Multiple Choice)
4.9/5  (28)
(28)
The synthesis of nitrogen monoxide proceeds by the reaction of ammonia with oxygen as shown in the following unbalanced reaction:
NH3(g) + O2(g) NO(g) + H2O(g)If O2 is being consumed at a rate of 32 mole/sec, what is the rate of NO production?
(Multiple Choice)
4.8/5  (47)
(47)
What is the rate law associated with the following mechanism:
HCl + HCl  H2Cl2
HCl + CH3CHCH2
H2Cl2
HCl + CH3CHCH2  CH3CHClCH3
H2Cl2 + CH3CHClCH3 CH3CHClCH3 +2 HCl
Net: HCl(g) + CH3CHCH2(g) CH3CHClCH3 (g)
CH3CHClCH3
H2Cl2 + CH3CHClCH3 CH3CHClCH3 +2 HCl
Net: HCl(g) + CH3CHCH2(g) CH3CHClCH3 (g)
(Multiple Choice)
4.9/5  (37)
(37)
The first-order rate constant for the decomposition of trioxane (C3H6O3) is known to be 3.05 x 10-4 s-1 at 519ºK. What is the half life of trioxane at 519ºK?
(Multiple Choice)
4.8/5  (37)
(37)
The activation energy for the high temperature conversion cyclopropane to propene is 270 kJ mol-1. At what temperature would the rate constant for this reaction be ten times that of 500oC?
(Short Answer)
4.9/5  (26)
(26)
Hydrogen peroxide decomposes according to the equation:
2 H2O2(aq) 2 H2O(l) + O2(g)
At 27°C and 1 atm, a 50.0 ml sample of hydrogen peroxide decomposes at a rate that produces 10.0 ml/sec of O2(g). Assuming ideal behavioura) Determine the moles of oxygen produced per second.b) Determine the change in molarity of H2O2 per second.
(Essay)
4.8/5  (38)
(38)
Heterogeneous catalysts are used in industrial processes because
I. they utilize more of the catalyst atoms.
II. it is easier to separate the products from the catalyst.
III. higher operating temperatures are readily obtained.
IV. they are more selective.Which of the above statements are true?
(Multiple Choice)
4.8/5  (39)
(39)
For a hypothetical reaction, the activation energy is Eact = 50.2 kJ/mol and it has an Arrhenius constant of 22.3 M-1s-1. Determine what the rate constant would be if the temperature was 400 C.
(Short Answer)
4.8/5  (34)
(34)
In which order do the following steps typically occur for reactions facilitated by heterogeneous catalysts:
I. Desorption of material
II. Adsorption on materials on catalyst surface
III. Reaction to form products
IV. Movement of bound species over catalyst surface
(Multiple Choice)
4.9/5  (45)
(45)
Showing 61 - 77 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)