Exam 10: An Introduction to Kinetics and Equilibrium
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
What is the Ksp of SrF2(s) if 0.073 g of SrF2 dissolves in 1.00 liter of solution?
(Multiple Choice)
4.9/5  (40)
(40)
The following reaction is at equilibrium. Which of the conditions listed below will cause the reaction to shift toward the products?
2 SO3(g) + 2 Cl2(g) 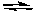 2 SO2Cl2(g)+ O2(g)
2 SO2Cl2(g)+ O2(g)
(Multiple Choice)
4.9/5  (36)
(36)
The following reaction was carried out at 250C with the initial concentration of NO2 being 0.70 M and no NO(g) or O2(g) initially present. At equilibrium the NO2 concentration was found to be 0.28 M. Calculate Kc for the reaction.
2NO2(g) 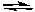 2NO(g) + O2(g)
2NO(g) + O2(g)
(Multiple Choice)
4.9/5  (41)
(41)
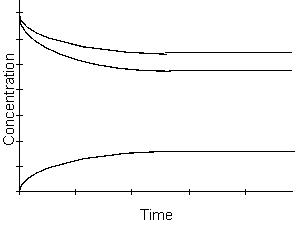 -What happens to the rate of the reaction as time passes in the equilibrium region of the graph above?
-What happens to the rate of the reaction as time passes in the equilibrium region of the graph above?
(Multiple Choice)
4.7/5  (32)
(32)
Use the following graph for answering
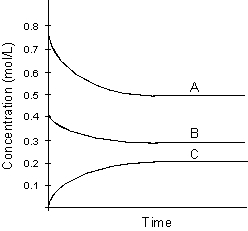 -Using the above graph determine the balanced chemical equation.
-Using the above graph determine the balanced chemical equation.
(Multiple Choice)
4.8/5  (32)
(32)
If 1.3 x 10 - 5 moles of AgCl will dissolve in 1.0 liter of water at 25-C, what is the Ksp of AgCl?
(Multiple Choice)
4.7/5  (32)
(32)
For the reaction below, 0.0500 moles of ethyl acetate are allowed to react with 0.200 moles of water. At equilibrium it is found that 0.0300 moles of acetic acid have been produced. How many moles of ethyl acetate remain at equilibrium?
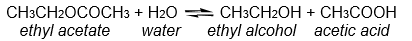
(Multiple Choice)
4.8/5  (47)
(47)
The following chemical reaction has reached equilibrium. Which of the changes listed below would cause the equilibrium to shift back toward the reactants?
N2(g) + 3 H2(g) 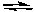 2 NH3(g) H = -92.2 kJ/molrxn
2 NH3(g) H = -92.2 kJ/molrxn
(Multiple Choice)
4.8/5  (49)
(49)
For the reaction:
CO(g) + Cl2(g) 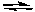 COCl2(g)
Kf is 1x 107 M-1-s-1 and kr = 2 x 102 s-1.
What is the equilibrium constant for the reaction?
COCl2(g)
Kf is 1x 107 M-1-s-1 and kr = 2 x 102 s-1.
What is the equilibrium constant for the reaction?
(Multiple Choice)
4.8/5  (27)
(27)
Use the following graph for answering
 -Which of the following will be true of the equilibrium constant, Kc, for the reaction described in the above graph?
-Which of the following will be true of the equilibrium constant, Kc, for the reaction described in the above graph?
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the NO, NO2, and O2 concentrations in the following gas-phase reaction at equilibrium at 200°C.
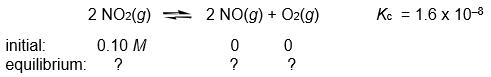
(Essay)
4.8/5  (55)
(55)
What would happen if F2 was removed from the following reaction while it was at equilibrium?
Cl2(g) + 3 F2(g) 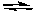 2 ClF3(g)
2 ClF3(g)
(Multiple Choice)
4.8/5  (23)
(23)
Calculate the equilibrium concentrations of SO3, SO2, and O2 if 0.100 moles of SO3 in a 10-L flask decompose to form SO2 and O2 at 500 K.
2 SO3(g) 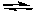 2 SO2(g) + O2(g) Kc = 1.4 x 10-11
2 SO2(g) + O2(g) Kc = 1.4 x 10-11
(Essay)
4.8/5  (31)
(31)
For the following reaction
2 C2H6(g) + 7 O2(g) ![For the following reaction 2 C<sub>2</sub>H<sub>6</sub>(g) + 7 O<sub>2</sub>(g) 4 CO<sub>2</sub>(g) + 6 H<sub>2</sub>O(g) If the reaction mixture initially contained all species at the same initial concentration, C, and if the concentration of O<sub>2</sub> at equilibrium was expressed as [O<sub>2</sub>] = C + 7 \Delta C , then the concentration of H<sub>2</sub>O at equilibrium would be correctly expressed as [H<sub>2</sub>O] =](https://storage.examlex.com/TB9692/11ee9da3_9e07_debe_9ff6_b37c2c390dbf_TB9692_11.jpg) 4 CO2(g) + 6 H2O(g)
If the reaction mixture initially contained all species at the same initial concentration, C, and if the concentration of O2 at equilibrium was expressed as [O2] = C + 7 C , then the concentration of H2O at equilibrium would be correctly expressed as [H2O] =
4 CO2(g) + 6 H2O(g)
If the reaction mixture initially contained all species at the same initial concentration, C, and if the concentration of O2 at equilibrium was expressed as [O2] = C + 7 C , then the concentration of H2O at equilibrium would be correctly expressed as [H2O] =
(Multiple Choice)
4.9/5  (30)
(30)
Showing 81 - 94 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)