Exam 10: An Introduction to Kinetics and Equilibrium
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
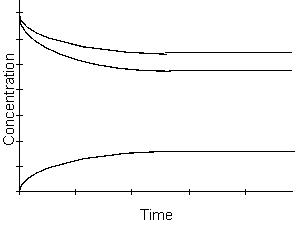 -Which of the following reactions could be described by the above diagram?
-Which of the following reactions could be described by the above diagram?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following initial concentrations of reactants and products will cause the reaction to proceed to the right in order to establish equilibrium?
2 NO2(g) 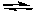 2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200
2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200  C)
C)
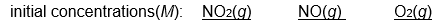
(Multiple Choice)
4.9/5  (43)
(43)
Calculate the COCl2, CO, and Cl2 concentrations when the following gas-phase reaction reaches equilibrium at 300°C.
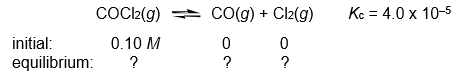
(Essay)
4.9/5  (44)
(44)
A solution is prepared in which the Pb2+ ion concentration is 0.020 M and the Br- ion concentration is 0.010 M. Which of the following statements is true given that Ksp for PbBr2 is 7.9 x 10-5?
(Multiple Choice)
4.7/5  (32)
(32)
What would happen to the extent of the decomposition of PCl5 at a given temperature if the pressure on the system were decreased by increasing the volume of the container at a constant temperature?
PCl5(g) 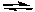 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
(Multiple Choice)
4.9/5  (35)
(35)
Assume that the equilibrium constants for the following reactions are known.
2 SO2(g) + O2(g) 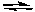 2 SO3(g) K1
2 CO(g) + O2(g)
2 SO3(g) K1
2 CO(g) + O2(g) 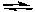 2 CO2(g) K2
What is the equilibrium constant for the following reaction?
2 SO2(g) + 2 CO2(g)
2 CO2(g) K2
What is the equilibrium constant for the following reaction?
2 SO2(g) + 2 CO2(g) 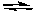 2 SO3(g) + 2 CO(g)
2 SO3(g) + 2 CO(g)
(Multiple Choice)
4.8/5  (36)
(36)
A 0.100 M sample of SO2 reacts with 0.060 M O2 to produce SO3 by the reaction shown below. There was no SO3 present initially. The concentration of O2 measured at equilibrium is found to be 0.020 M. What are the equilibrium concentrations of SO2 and SO3?
2 SO2(g) + O2(g) 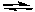 2 SO3(g)
2 SO3(g)
(Multiple Choice)
4.8/5  (36)
(36)
What is the concentration of Ag+ (mol/L) ion that results from saturating a 0.10 M NaBr solution with AgBr?
The Ksp of AgBr is 5.0 x 10-13.
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following equations describes the relationship between the solubility product for Ag2SO4 and the solubility of this compound?
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the concentrations of all reactants and products when a mixture
that is initially 0.10 M in N2, H2, and NH3 comes to equilibrium at 600 K.
N2(g) + 3 H2(g) 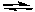 2 NH3(g) Kc = 0.045
2 NH3(g) Kc = 0.045
(Essay)
4.8/5  (35)
(35)
The following reactions are at equilibrium. Beside each reaction is a change that is made to the system which may cause a shift in equilibrium. Which of the reactions will shift its equilibrium toward the products?
(Multiple Choice)
4.9/5  (39)
(39)
What is the solubility in moles/L of CaF2 in water given that the Ksp is 4.0 x 10 - 11?
(Multiple Choice)
4.8/5  (40)
(40)
Assume an equilibrium constant Kc = 1.7 x 10-2 for the reaction:
N2(g) + 3 H2(g) 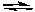 2 NH3(g)
Calculate (to a first approximation) the equilibrium concentrations of NH3, H2 and N2 when 0.10 moles per liter each of N2 and H2 are mixed and allowed to react to equilibrium.
2 NH3(g)
Calculate (to a first approximation) the equilibrium concentrations of NH3, H2 and N2 when 0.10 moles per liter each of N2 and H2 are mixed and allowed to react to equilibrium.
(Essay)
5.0/5  (38)
(38)
If the Ksp of PbBr2 is 8.9 x 10-6, what is the solubility of PbBr2(s) in moles/L?
(Multiple Choice)
5.0/5  (36)
(36)
What is the correct solubility constant expression for AgCl(s)?
(Multiple Choice)
4.8/5  (39)
(39)
What is the correct solubility constant expression for PbSO4?
(Multiple Choice)
4.8/5  (30)
(30)
For the reaction shown below, at equilibrium
I2(g) + Br2(g) 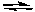 2IBr(g) Kc = 1.1 x 102
2IBr(g) Kc = 1.1 x 102
(Multiple Choice)
4.8/5  (34)
(34)
What is the effect of increasing the temperature on the following exothermic reaction?
2 CO(g) + O2(g) 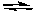 2 CO2(g)
2 CO2(g)
(Multiple Choice)
4.9/5  (49)
(49)
The following reactions are at equilibrium. Which of the reactions will shift its equilibrium toward the reactant side if the pressure is increased while holding the temperature constant?
(Multiple Choice)
4.7/5  (34)
(34)
Showing 61 - 80 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)