Exam 10: An Introduction to Kinetics and Equilibrium
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
A 50.0 mL solution of 0.015 M of Ca(NO3)2 is added to 50.0 mL of 0.010 M NaF. Write a net ionic equation that shows the formation of a precipitate of CaF2(s). Given that the Ksp of CaF2(s) is 4.0 x 10-11, will a precipitate form from this mixture?
(Essay)
4.9/5  (42)
(42)
Which is the correct reaction for the solubility constant expression (Ksp) of CaF2(s)?
(Multiple Choice)
4.8/5  (37)
(37)
What is the effect of increasing the temperature on the following reaction?
2 CO(g) + O2(g) 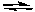 2 CO2(g) + heat
2 CO2(g) + heat
(Multiple Choice)
4.9/5  (38)
(38)
Suppose that the reaction quotient, Qc, for a chemical reaction was found to be close to unity (Qc 1). Given this information we can accurately predict
(Multiple Choice)
4.9/5  (35)
(35)
What is the fluoride ion concentration in a saturated solution of SrF2 if the strontium ion concentration is 5.8 x 10 - 4 M?
(Multiple Choice)
4.8/5  (32)
(32)
What is the relationship between the changes in the concentration of NO2 and O2 as the following reaction comes to equilibrium?
2 NO2(g) 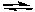 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
(Multiple Choice)
4.7/5  (40)
(40)
If 6.5 x 10 - 5 moles of Ag2CrO4 will dissolve in 1.0 liter of water at 25  C, what is the Ksp of Ag2CrO4?
C, what is the Ksp of Ag2CrO4?
(Multiple Choice)
4.9/5  (32)
(32)
What is the correct solubility constant expression of Ba3(PO4)2?
(Multiple Choice)
4.8/5  (36)
(36)
One of the products of the reaction between NO2 and CO is CO2. By writing the equation for the reaction (Hint: the coefficients for all reactants and products are 1), decide whether an increase in pressure:
(Multiple Choice)
4.9/5  (30)
(30)
What would happen if Cl2 is added to the following system at equilibrium?
H2(g) + Cl2(g) 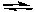 2 HCl(g)
2 HCl(g)
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following changes will shift the following equilibrium to the right?
AgCl(s) 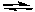 Ag+(aq) + Cl-(aq) H = 65.4 kJ/molrxn
Ag+(aq) + Cl-(aq) H = 65.4 kJ/molrxn
(Multiple Choice)
4.7/5  (37)
(37)
For the reaction
2 NOCl(g) 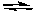 2 NO(g) + Cl2 Kc = 5.6 x 10-6 (at 400 K)
What is the relationship between the magnitude of the changes in the concentrations of NOCl and Cl2 as this reaction comes to equilibrium?
2 NO(g) + Cl2 Kc = 5.6 x 10-6 (at 400 K)
What is the relationship between the magnitude of the changes in the concentrations of NOCl and Cl2 as this reaction comes to equilibrium?
(Multiple Choice)
4.7/5  (45)
(45)
The equilibrium constant for the following reaction becomes larger as the temperature at which the reaction is run increases.
2 NOCl(g) 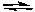 2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 at 400 K
What does this tell us about the reaction?
2 NO(g) + Cl2(g) Kc = 5.6 x 10-6 at 400 K
What does this tell us about the reaction?
(Multiple Choice)
4.9/5  (32)
(32)
If at any moment in time, we find that Qc is larger than Kc for the reaction
Cl2(g) + 3 F2(g) 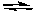 2 ClF3(g)
We can conclude that:
2 ClF3(g)
We can conclude that:
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following equations correctly describes the relationship between the concentrations of Sr2+ and PO43- ions in a saturated solution of Sr3(PO4)2(s)?
(Multiple Choice)
4.9/5  (38)
(38)
What would happen to the extent of the decomposition of PCl5 at a given temperature if the pressure on the system were decreased?
PCl5(g) 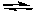 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
(Multiple Choice)
4.9/5  (44)
(44)
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 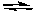 2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-When solving a problem with the initial conditions outlined below, we rearrange the problem to give an intermediate set of conditions where the concentration of one of the reactants or products is equal to zero.
Fe(CO)5
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-When solving a problem with the initial conditions outlined below, we rearrange the problem to give an intermediate set of conditions where the concentration of one of the reactants or products is equal to zero.
Fe(CO)5 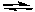 Fe(CO)4 + CO
Initial: 0.10 M 0.10 M 0.005 M
Our choice of whether to push the reaction all the way to the right or all the way to the left is determined by which of the following goals?
Fe(CO)4 + CO
Initial: 0.10 M 0.10 M 0.005 M
Our choice of whether to push the reaction all the way to the right or all the way to the left is determined by which of the following goals?
(Multiple Choice)
4.7/5  (44)
(44)
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 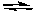 2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-Starting with only 0.10 M H2O(g), which equation correctly shows the equilibrium concentrations of the reactants and products? ( C represents the change in concentration of O2(g))
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-Starting with only 0.10 M H2O(g), which equation correctly shows the equilibrium concentrations of the reactants and products? ( C represents the change in concentration of O2(g))
(Multiple Choice)
4.8/5  (40)
(40)
Hidden Assumptions that make Equilibrium Calculations Easier What Do We Do When the Approximation Fails?
based on the following reaction:
2H2O(g) 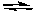 2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-The equation below solves for the change in concentration for the given reaction assuming the initial concentration of H2O(g) is 0.10 M. Which equation will result if it is assumed that the change will be much smaller than the initial concentration of H2O(g)?
2H2(g) + O2(g)
Kc = 2 x 10-42 (at 25oC)
-The equation below solves for the change in concentration for the given reaction assuming the initial concentration of H2O(g) is 0.10 M. Which equation will result if it is assumed that the change will be much smaller than the initial concentration of H2O(g)?
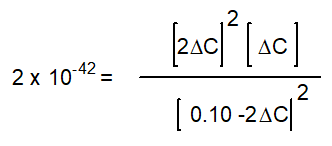
(Multiple Choice)
4.7/5  (44)
(44)
Showing 21 - 40 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)