Exam 10: An Introduction to Kinetics and Equilibrium
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
The addition of I2(g) to a fixed volume container with I2(g), H2(g), and HI(g) at equilibrium at a given temperature would cause
H2(g) + I2(g) 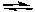 2 HI(g)
2 HI(g)
(Multiple Choice)
4.8/5  (34)
(34)
A solution is prepared in which the Ag+ ion concentration is 2.5 x 10-3 M and the Cl- ion concentration is 3.2 x 10-4 M. Which of the following statements is true given that the Ksp for AgCl is 1.8 x 10-10?
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following equations correctly describes the relationship between the concentrations of Pb2+ and Br- ions in a saturated solution of PbBr2(s)?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following statements correctly describes the following reaction?
2 NH3(g) 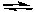 N2(g) + 3 H2(g) H = 92.2 kJ
N2(g) + 3 H2(g) H = 92.2 kJ
(Multiple Choice)
4.8/5  (36)
(36)
A solution is 0.10 M in Ca(NO3)2. What would the concentration of NaOH need to be for a precipitate of Ca(OH)2 to form?
Ksp Ca(OH)2 = 5.5 x 10-6.
(Multiple Choice)
4.9/5  (44)
(44)
What would be the effect of decreasing the pressure on the following reaction after it reaches equilibrium?
2 NO2(g) 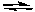 2 NO(g) + O2(g)
2 NO(g) + O2(g)
(Multiple Choice)
4.9/5  (27)
(27)
Assume that the reaction quotient, Qc, for the following reaction at 25°C is 1.0 x 10-8
2 NO2(g) 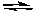 2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
From this we can conclude:
2 NO(g) + O2(g) Kc = 7.4 x 10-16 (at 25°C)
From this we can conclude:
(Multiple Choice)
4.7/5  (34)
(34)
What is the Ksp of PbBr2 if the concentration of PbBr2(aq) in a saturated solution is 1.3 x 10-2 moles/L?
(Multiple Choice)
4.8/5  (31)
(31)
What would happen if O2 were removed from the following system at equilibrium at 25°C?
2 NO2(g) 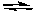 2 NO(g) + O2(g) Kc = 7.4 x 10-1 at 25oC
2 NO(g) + O2(g) Kc = 7.4 x 10-1 at 25oC
(Multiple Choice)
4.9/5  (39)
(39)
Of the compounds in the table below, _______ is the soluble.

(Multiple Choice)
4.8/5  (29)
(29)
Which one of the following graphs best represents the relationship between the concentration of reactants and products with respect to time for the following chemical reaction?
2 NO2(g) 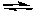 2 NO(g) + O2(g)
2 NO(g) + O2(g)
(Multiple Choice)
4.9/5  (38)
(38)
Approximately how many grams of Ag2CO3 will dissolve in 1.0 liter of a solution that is 0.10 M in Na2CO3?
The Ksp of Ag2CO3 is 6.2 x 10-12.
(Multiple Choice)
5.0/5  (38)
(38)
Ag2SO4(s) is in equilibrium with silver and sulfate ions in solution:
Ag2SO4(s) 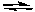 2Ag+(aq) + SO42-(aq)
Which of the following will not increase the amount of solid Ag2SO4(s) present?
2Ag+(aq) + SO42-(aq)
Which of the following will not increase the amount of solid Ag2SO4(s) present?
(Multiple Choice)
4.8/5  (46)
(46)
If a solution has a Mg2+ ion concentration of 5.0 x 10-3 M and an OH- concentration of 0.010 M, will a precipitate form? Ksp Mg(OH)2 = 1.7 x 10-6.
(Essay)
4.8/5  (36)
(36)
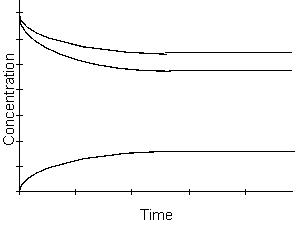 -Which of the following will be true concerning the Kc of the reaction described by the graph above?
-Which of the following will be true concerning the Kc of the reaction described by the graph above?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following equilibria would not be affected by changes in pressure?
(Multiple Choice)
4.8/5  (37)
(37)
The following reaction was carried out:
Ni(CO)4 + PPh3(g) PPh3Ni(CO)3 + CO
Over 200 seconds, the concentration of Ni(CO)4 dropped from 10 M to 2.5 M. What is the average rate of the reaction in M/s over this time period?
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following equations describes the relationship between the solubility product for AgCl and the solubility of this compound?
(Multiple Choice)
4.8/5  (27)
(27)
What is the solubility in moles per liter of AgBr in water given that the Ksp is 5.0 x 10-13?
(Multiple Choice)
4.8/5  (34)
(34)
Describe the relationship between the rate constants kf and kr for the following one step reaction at equilibrium.
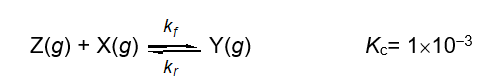
(Multiple Choice)
4.9/5  (41)
(41)
Showing 41 - 60 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)