Exam 7: Electron Configurations and the Periodic Table
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
Which type(s) of atomic orbital(s) has (have) two lobes of electron density?
Free
(Multiple Choice)
5.0/5  (25)
(25)
Correct Answer:
B
Which ion has the largest ionic radius?
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
C
Which atom or ion does not have the electron configuration [Ne]3s23p6?
(Multiple Choice)
4.9/5  (27)
(27)
Determine the energy of a photon that has a wavelength of 714 nm. The speed of light is 3.00 × 108 m/s and h = 6.63 × 10-34 J s.
(Multiple Choice)
4.9/5  (35)
(35)
Atomic radii _____________ going from left to right across a row of the periodic table.
(Short Answer)
4.9/5  (33)
(33)
Determine the energy of a photon that has a frequency of 5.23 × 1014 Hz. Given: h = 6.63 × 10-34 J s.
(Multiple Choice)
4.9/5  (42)
(42)
The first ionization energy is the amount of energy required to remove one ____________ from a neutral atom.
(Short Answer)
4.9/5  (28)
(28)
What is the correct shorthand notation for the electron configuration given? 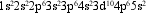
(Multiple Choice)
4.8/5  (34)
(34)
Light has a wavelength of 444 nm. What is its frequency? The speed of light is 3.00 × 108 m/s.
(Multiple Choice)
4.7/5  (37)
(37)
What is the phenomenon that occurs when excited gaseous elements emit only a few colored lines?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 1 - 20 of 64
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)