Exam 7: Electron Configurations and the Periodic Table
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
The d orbitals occur in groups of ____ and hold up to ____ electrons.
(Multiple Choice)
4.8/5  (35)
(35)
What is the phenomenon that occurs when certain metals emit electrons when illuminated by particular wavelengths of light?
(Multiple Choice)
4.8/5  (39)
(39)
Light has a wavelength of 582 nm. What is its frequency? The speed of light is 3.00 × 108 m/s.
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is not a characteristic of the Bohr model of the atom?
(Multiple Choice)
4.9/5  (41)
(41)
Each electron in an atom or ion must have a unique set of four _____________.
(Short Answer)
4.9/5  (31)
(31)
Which statement regarding the Bohr model of the atom is true?
(Multiple Choice)
4.7/5  (50)
(50)
Which word or phrase least applies to the quantum number represented by the symbol n?
(Multiple Choice)
4.9/5  (39)
(39)
Light has a frequency of 7.21 × 1013 hertz. What is its wavelength? The speed of light is 3.00 × 108 m/s.
(Multiple Choice)
4.7/5  (40)
(40)
Arrange these elements in order of increasing ionization energy. 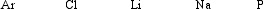
(Multiple Choice)
4.9/5  (40)
(40)
Showing 21 - 40 of 64
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)