Exam 2: Atoms and Elements
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
Which of the following represent a pair of isotopes? 
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
D
Suppose the isotopic ratio of the two boron isotopes 10B (10.013 amu) and 11B (11.009 amu) in a sample has been altered from the ratio found in nature and now contains 58.73% 10B in the sample. Determine the atomic weight of this sample of boron.
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
D
Which element has an atomic number of 35 and mass number of 80?
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
E
Three elements that are likely to have similar chemical and physical properties are:
(Multiple Choice)
5.0/5  (33)
(33)
Write the atomic symbol for an atom that contains 24 protons, 24 electrons and 26 neutrons.
(Short Answer)
4.8/5  (43)
(43)
Discuss what role Rutherford's gold foil experiment had in leading to the modern model of the atom.
(Essay)
4.7/5  (33)
(33)
Assume that only two isotopes of argon, 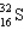 and
and 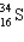 exist in nature. If this were true, the atomic weight of sulfur would fall most closely within which range?
exist in nature. If this were true, the atomic weight of sulfur would fall most closely within which range?
(Multiple Choice)
4.8/5  (43)
(43)
Which sample contains the same number of atoms as 175.0 g of cadmium (Cd)?
(Multiple Choice)
4.7/5  (27)
(27)
Which of the following is the most correct for the cathode ray experiments?
(Multiple Choice)
4.8/5  (32)
(32)
An element that has 32 protons and 52 neutrons has an approximate atomic weight of:
(Multiple Choice)
4.8/5  (34)
(34)
Which element can be classified as an alkaline earth metal?
(Multiple Choice)
4.9/5  (40)
(40)
An element containing 26 protons, 26 electrons and 30 neutrons will have the symbol:
(Multiple Choice)
4.9/5  (42)
(42)
Electromagnetic radiation having a wavelength of 475 nm is in the blue region of the visible spectrum. Converting this wavelength into centimeters yields:
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)