Exam 8: Electron Configurations and Periodicity
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Who was the first chemist to recognize patterns in chemical properties of the elements?
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
B
An atom of which of the following elements has the highest fourth ionization energy?
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
A
Fe has ____ that is(are)unpaired in its d orbitals.
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
D
The statement that the first ionization energy for an oxygen atom is lower than the first ionization energy for a nitrogen atom is
(Multiple Choice)
4.9/5  (32)
(32)
Rank the following ions in order of increasing first ionization energy: O2-,Mg2+,F-,Na+.
(Multiple Choice)
5.0/5  (45)
(45)
Which element is found in the s-block of the periodic table?
(Multiple Choice)
4.8/5  (33)
(33)
An atom of which of the following elements has the smallest first ionization energy?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following have one or more filled d subshells in their ground state electron configuration?
(Multiple Choice)
4.8/5  (33)
(33)
The change in energy for which of the following processes represents the first ionization energy of iodine?
(Multiple Choice)
4.9/5  (39)
(39)
What is the ground-state electron configuration of terbium (Tb)?
(Multiple Choice)
4.9/5  (28)
(28)
Sodium and potassium have similar chemical and physical properties.This is best explained by the fact that both elements
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following orbital diagrams violates the Pauli exclusion principle?
1s 2s 2p
(Multiple Choice)
4.8/5  (34)
(34)
The statement that "the lowest-energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate orbitals" is known as
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following statements concerning the periodic table is incorrect?
(Multiple Choice)
4.9/5  (29)
(29)
A section of the periodic table with all identification features removed is shown below.
V
W
X
Y
Z
Which element has the smallest atomic radius?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following electron configurations corresponds to the ground state of an atom of a transition element?
(Multiple Choice)
4.9/5  (45)
(45)
What is the maximum number of electrons in an atom that have the set of quantum numbers n= 2 and l= 1?
(Multiple Choice)
4.8/5  (34)
(34)
If the electron could have a third spin state (that is, 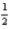 ,-
,- 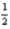 ,and 0),what would be the ground-state electron configuration of carbon?
,and 0),what would be the ground-state electron configuration of carbon?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 1 - 20 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)