Exam 19: Electrochemistry
Exam 1: Chemistry and Measurement111 Questions
Exam 2: Atoms, molecules, and Ions149 Questions
Exam 3: Calculations With Chemical Formulas and Equations139 Questions
Exam 4: Chemical Reactions159 Questions
Exam 5: The Gaseous State104 Questions
Exam 6: Thermochemistry119 Questions
Exam 7: Quantum Theory of the Atom68 Questions
Exam 8: Electron Configurations and Periodicity100 Questions
Exam 9: Ionic and Covalent Bonding125 Questions
Exam 10: Molecular Geometry and Chemical Bonding Theory101 Questions
Exam 11: States of Matter; Liquids and Solids123 Questions
Exam 12: Solutions119 Questions
Exam 13: Rates of Reaction113 Questions
Exam 14: Chemical Equilibrium97 Questions
Exam 15: Acids and Bases83 Questions
Exam 16: Acid-Base Equilibria148 Questions
Exam 17: Solubility and Complex-Ion Equilibria115 Questions
Exam 18: Thermodynamics and Equilibrium94 Questions
Exam 19: Electrochemistry122 Questions
Exam 20: Nuclear Chemistry90 Questions
Exam 21: Chemistry of the Main-Group Metals121 Questions
Exam 22: The Transition Elements and Coordination Compounds75 Questions
Exam 23: Organic Chemistry79 Questions
Exam 24: Polymer Materials: Synthetic and Biological56 Questions
Select questions type
Balance the following half-reaction occurring in acidic solution.
NO3-(aq)→ 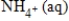
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
A
When the following oxidation-reduction reaction in acidic solution is balanced,what is the lowest whole-number coefficient for H+,and on which side of the balanced equation should it appear?
S2O82-(aq)+ NO(g)→ SO42-(aq)+ NO3-(aq)
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
D
A fuel cell designed to react grain alcohol with oxygen has the following net reaction:
C2H5OH(l)+ 3O2(g)→ 2CO2(g)+ 3H2O(l)
The maximum work that 1 mol of alcohol can yield by this process is 1320 kJ.What is the theoretical maximum voltage that this cell can achieve?
Free
(Multiple Choice)
4.7/5  (46)
(46)
Correct Answer:
A
Given:
Mn2+(aq)+ 2e-  Mn(s); E° = -1.18 V
Cu2+(aq)+ 2e-
Mn(s); E° = -1.18 V
Cu2+(aq)+ 2e-  Cu(s); E° = 0.34 V
Cr2O72-(aq)+ 14H+(aq)+ 6e-
Cu(s); E° = 0.34 V
Cr2O72-(aq)+ 14H+(aq)+ 6e-  2Cr3+(aq)+ 7H2O(l); E° = 1.33 V
Which of the following species is the strongest reducing agent?
2Cr3+(aq)+ 7H2O(l); E° = 1.33 V
Which of the following species is the strongest reducing agent?
(Multiple Choice)
4.9/5  (34)
(34)
For a galvanic cell using Fe | Fe2+(1.0 M)and Pb | Pb2+(1.0 M)half-cells,which of the following statements is correct?
Fe2+(aq)+ 2e- → Fe(s); E° = -0.41 V
Pb2+(aq)+ 2e- → Pb(s); E° = -0.13 V
(Multiple Choice)
5.0/5  (30)
(30)
What is the cell reaction for the following voltaic cell?
Cr(s)| Cr3+(aq)|| Br-(aq)| Br2(g)| Pt(s)
(Multiple Choice)
4.8/5  (36)
(36)
A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 3.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.10 M and 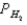 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at 25oC?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is E for the cell at 25oC?
(Multiple Choice)
4.8/5  (36)
(36)
What is one of the major products in the electrolysis of a saturated aqueous sodium chloride solution?
Reduction Half-Reaction
E° (V)
Na+(aq)+ e-  Na(s) -2.71
Mg2+(aq)+ 2e-
Na(s) -2.71
Mg2+(aq)+ 2e-  Mg(s) -2.37
2H2O(l)+ 2e-
Mg(s) -2.37
2H2O(l)+ 2e-  H2(g)+ 2OH-(aq) -0.83
O2(g)+ 4H+(aq)+ 4e-
H2(g)+ 2OH-(aq) -0.83
O2(g)+ 4H+(aq)+ 4e-  2H2O(l)1.23
Cl2(g)+ 2e-
2H2O(l)1.23
Cl2(g)+ 2e-  2Cl-(aq)
1.36
2Cl-(aq)
1.36
(Multiple Choice)
4.8/5  (19)
(19)
Given:
Li+(aq)+ e-  Li(s); E° = -3.04 V
Mg2+(aq)+ 2e-
Li(s); E° = -3.04 V
Mg2+(aq)+ 2e-  Mg(s); E° = -2.38 V
Fe2+(aq)+ 2e-
Mg(s); E° = -2.38 V
Fe2+(aq)+ 2e-  Fe(s); E° = -0.41 V
Ag+(aq)+ e-
Fe(s); E° = -0.41 V
Ag+(aq)+ e-  Ag(s); E° = 0.80 V
Br2(l)+ 2e-
Ag(s); E° = 0.80 V
Br2(l)+ 2e-  2Br-(aq); E° = 1.07 V
Which of the following species is the best oxidizing agent?
2Br-(aq); E° = 1.07 V
Which of the following species is the best oxidizing agent?
(Multiple Choice)
5.0/5  (34)
(34)
In the following electrochemical cell,what is the reduction half reaction?
Mn(s)| Mn2+(aq)|| Fe3+(aq),Fe2+(aq)| Pt(s)
(Multiple Choice)
5.0/5  (37)
(37)
The process of producing a chemical change in an electrolytic cell is called _____.
(Multiple Choice)
4.9/5  (31)
(31)
What is the cell reaction for the following electrochemical cell?
Zn | Zn2+(aq)|| Sc3+(aq)| Sc
(Multiple Choice)
4.8/5  (30)
(30)
A piece of iron half-immersed in a sodium chloride solution will corrode more rapidly than a piece of iron half-immersed in pure water,because
(Multiple Choice)
4.9/5  (31)
(31)
The cell potential of an electrochemical cell with the cell reaction
2Al(s)+ 3Zn2+(aq)→ 3Zn(s)+ 2Al3+(aq)
Is 1.607 V.What is the maximum electrical work obtainable from this cell when 2.0 mol of Al is consumed?
(Multiple Choice)
5.0/5  (39)
(39)
A voltaic cell is made by placing an iron electrode in a compartment in which the Fe2+ concentration is 2.0 × 10-5 M and by placing a Pt electrode in the other compartment,in which the H+ concentration is 3.4 M and 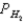 = 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is the value of E° for this cell,and which electrode is the anode?
= 1.00 atm.The Fe2+/Fe half-cell reduction potential is -0.41 V,and the H+/H2 half-cell reduction potential is 0.00 V.What is the value of E° for this cell,and which electrode is the anode?
(Multiple Choice)
4.8/5  (32)
(32)
An alkaline dry cell is similar to Leclanché cell,but it has _____ in place of ammonium chloride.
(Multiple Choice)
4.7/5  (37)
(37)
If the cell is initially at standard-state conditions,which of the following statements is true? 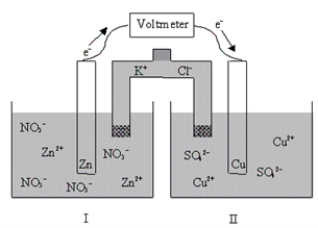 Zn2+(aq)+ 2e-
Zn2+(aq)+ 2e-  Zn(s); E° = -0.76 V
Cu2+(aq)+ 2e-
Zn(s); E° = -0.76 V
Cu2+(aq)+ 2e-  Cu(s); E° = 0.34 V
Cu(s); E° = 0.34 V
(Multiple Choice)
4.7/5  (40)
(40)
The _____ has zinc can as the anode; a graphite rod in the center,surrounded by a paste of manganese dioxide,ammonium and zinc chlorides,and carbon black,is the cathode.
(Multiple Choice)
4.8/5  (23)
(23)
Given:
W3+(aq)+ 3e-  W(s); E° = 2.72 V
Pb2+(aq)+ 2e-
W(s); E° = 2.72 V
Pb2+(aq)+ 2e-  Pb(s); E° = -0.13 V
Ni2+(aq)+ 2e-
Pb(s); E° = -0.13 V
Ni2+(aq)+ 2e-  Ni(s); E° = -0.23 V
Cd2+(aq)+ 2e-
Ni(s); E° = -0.23 V
Cd2+(aq)+ 2e-  Cd(s); E° = -0.40 V
Zn2+(aq)+ 2e-
Cd(s); E° = -0.40 V
Zn2+(aq)+ 2e-  Zn(s); E° = -0.76 V
Al3+(aq)+ 3e-
Zn(s); E° = -0.76 V
Al3+(aq)+ 3e-  Al(s); E° = -1.66 V
Mg2+(aq)+ 2e-
Al(s); E° = -1.66 V
Mg2+(aq)+ 2e-  Mg(s); E° = -2.38 V
Under standard-state conditions,which of the following metals will reduce W3+ to W but will not reduce Ni2+ to Ni?
Mg(s); E° = -2.38 V
Under standard-state conditions,which of the following metals will reduce W3+ to W but will not reduce Ni2+ to Ni?
(Multiple Choice)
4.7/5  (36)
(36)
Given:
Zn2+(aq)+ 2e-  Zn(s); E° = -0.76 V
Cu2+(aq)+ 2e-
Zn(s); E° = -0.76 V
Cu2+(aq)+ 2e-  Cu(s); E° = 0.34 V
Cr2O72-(aq)+ 14H+(aq)+ 6e-
Cu(s); E° = 0.34 V
Cr2O72-(aq)+ 14H+(aq)+ 6e-  2Cr3+(aq)+ 7H2O(l); E° = 1.33 V
Which of the following species is the strongest oxidizing agent?
2Cr3+(aq)+ 7H2O(l); E° = 1.33 V
Which of the following species is the strongest oxidizing agent?
(Multiple Choice)
4.8/5  (25)
(25)
Showing 1 - 20 of 122
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)