Exam 9: Molecular Geometry and Bonding Theories
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
How many unhybridized p atomic orbital(s)are found in an sp-hybridized carbon atom?
(Multiple Choice)
4.8/5  (36)
(36)
Structural changes around a ________ bond in the retinal portion of the rhodopsin molecule trigger the chemical reactions that result in vision.
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following molecules or ions will exhibit delocalized bonding?
NO2- NH4+ N3-
(Multiple Choice)
4.8/5  (37)
(37)
There are ________ σ bonds and ________ π bonds in H3C-CH2-CH  CH-CH2-C
CH-CH2-C  CH.
CH.
(Multiple Choice)
4.8/5  (29)
(29)
The bond order of a homonuclear diatomic molecule can be decreased by ________.
(Multiple Choice)
4.8/5  (26)
(26)
In the valence shell of an atom there are six electron domains.They will be arranged in a(n)________ geometry.
(Short Answer)
4.7/5  (31)
(31)
The basis of the VSEPR model of molecular bonding is ________.
(Multiple Choice)
4.8/5  (43)
(43)
An antibonding π orbital contains a maximum of ________ electrons.
(Multiple Choice)
4.8/5  (32)
(32)
Using the VSEPR model,the molecular geometry of the central atom in IF5 is ________.
(Multiple Choice)
5.0/5  (39)
(39)
Which of the following molecules or ions have various resonance structures?
CO2 O3 CO32-
(Multiple Choice)
4.9/5  (30)
(30)
According to molecular orbital theory,the greater the ________ ,the shorter the bond length.
(Short Answer)
4.7/5  (28)
(28)
The bond angles marked a,b,and c in the molecule below are about ________,________,and ________,respectively. 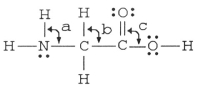
(Multiple Choice)
4.9/5  (34)
(34)
Using the VSEPR model,the electron-domain geometry of the central atom in ClO3- is ________.
(Multiple Choice)
5.0/5  (39)
(39)
In comparing the same two atoms bonded together,the ________ the bond order,the ________ the bond length,and the ________ the bond energy.
(Multiple Choice)
4.8/5  (34)
(34)
The electron-domain geometry and molecular geometry of the nitrite ion are ________ and ________,respectively.
(Multiple Choice)
4.9/5  (36)
(36)
According to molecular orbital theory,the bond order in a Be2 molecule is ________.
(Multiple Choice)
4.9/5  (36)
(36)
The ________ hydrogen orbital overlaps with the ________ bromide orbital in HBr.
(Short Answer)
4.9/5  (37)
(37)
Showing 61 - 80 of 183
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)