Exam 9: Molecular Geometry and Bonding Theories
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
________ hybrid orbitals are used for bonding by Xe in the XeF4 molecule.
(Multiple Choice)
4.8/5  (37)
(37)
The electron-domain geometry of the AsF6- ion is octahedral.The hybrid orbitals used by the As atom for bonding are ________ orbitals.
(Multiple Choice)
4.7/5  (40)
(40)
In molecular orbital theory,the bond order of the He-He bond in He2 is ________.
(Multiple Choice)
4.9/5  (40)
(40)
The Cl-Si-Cl bond angle in the SiCl2F2 molecule is approximately ________.
(Multiple Choice)
4.8/5  (32)
(32)
There are ________ σ and ________ π bond(s)in the H2C=CH2 molecule.
(Multiple Choice)
4.8/5  (32)
(32)
The hybridizations of bromine in BrF5 and of arsenic in AsF5 are ________ and ________,respectively.
(Multiple Choice)
4.9/5  (36)
(36)
According to MO theory,overlap of two p atomic orbitals produces ________.
(Multiple Choice)
4.9/5  (29)
(29)
In order to produce sp3 hybrid orbitals,________ s atomic orbital(s)and ________ p atomic orbital(s)must be mixed.
(Multiple Choice)
4.8/5  (32)
(32)
The hybridization of nitrogen in the H-C  N: molecule is ________.
N: molecule is ________.
(Multiple Choice)
4.7/5  (31)
(31)
The π bond in ethylene,H2C  CH2,results from the overlap of ________.
CH2,results from the overlap of ________.
(Multiple Choice)
4.9/5  (33)
(33)
The hybridization of the oxygen atom labeled x in the structure below is ________. 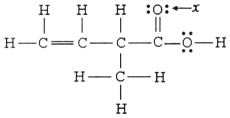
(Multiple Choice)
4.9/5  (34)
(34)
Construct a molecular orbital diagram for a Li2 molecule.According to molecular orbital theory,the σ1s orbital is ________ and the σ1s* orbital is ________.
(Multiple Choice)
4.8/5  (34)
(34)
Mixing one s atomic orbital and one p atomic orbital gives rise to ________.
(Multiple Choice)
4.9/5  (42)
(42)
Showing 161 - 180 of 183
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)