Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Cesium-137 undergoes beta decay and has a half-life of 30.0 years.How many beta particles are emitted by a 14.0-g sample of cesium-137 in three minutes?
(Multiple Choice)
4.8/5  (28)
(28)
The beta decay of cesium-137 has a half-life of 30.0 years.How many years must pass to reduce a 30 mg sample of cesium 137 to 5.2 mg?
(Multiple Choice)
4.8/5  (39)
(39)
The mass of a proton is 1.00728 amu and that of a neutron is 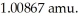 What is the mass defect (in amu)of a 57Ni nucleus? (The mass of a nickel-57 nucleus is
What is the mass defect (in amu)of a 57Ni nucleus? (The mass of a nickel-57 nucleus is 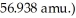
(Multiple Choice)
4.8/5  (45)
(45)
Conversion of one nucleus into another was first demonstrated in 1919 by ________.
(Short Answer)
4.8/5  (41)
(41)
If we start with 1.000 g of cobalt-60,0.400 g will remain after 7.00 yr.This means that the 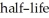 of
of 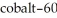 is ________ yr.
is ________ yr.
(Multiple Choice)
4.8/5  (31)
(31)
Radioactive seeds that are implanted into a tumor are coated with ________ to stop alpha and beta ray penetration.
(Short Answer)
4.8/5  (37)
(37)
Which one of the following is a correct representation of beta particle?
(Multiple Choice)
4.9/5  (35)
(35)
The belt of nuclear stability ends with the element ________.
(Multiple Choice)
4.9/5  (35)
(35)
On average,________ neutrons are produced by every fission of a uranium-235 nucleus.
(Multiple Choice)
4.7/5  (40)
(40)
Which one of the following is a correct representation of a positron?
(Multiple Choice)
4.9/5  (27)
(27)
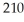 Pb has a half-life of 22.3 years and decays to produce
Pb has a half-life of 22.3 years and decays to produce 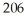 Hg.If you start with
Hg.If you start with 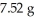 of
of 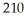 Pb,how many grams of
Pb,how many grams of 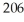 Hg will you have after 15.8 years?
Hg will you have after 15.8 years?
(Multiple Choice)
4.8/5  (39)
(39)
The beta decay of cesium-137 has a half-life of 30.0 years.How many years must pass to reduce a 25 mg sample of cesium 137 to 8.7 mg?
(Multiple Choice)
4.8/5  (38)
(38)
In the nuclear transmutation represented by
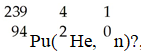 ,what is the product?
,what is the product?
(Multiple Choice)
4.9/5  (37)
(37)
What isotope of what element is produced if uranium-238 undergoes alpha decay?
(Short Answer)
4.9/5  (26)
(26)
The half-life of 223Ra is 11.4 days.How much of a 200.0 mg sample remains after 600 hours?
(Multiple Choice)
4.9/5  (29)
(29)
When an atom of an element undergoes beta decay,its proton count will change by ________ and its neutron count will change by ________.
(Multiple Choice)
4.8/5  (40)
(40)
When two atoms of 2H are fused to form one atom of 4He,the total energy evolved is 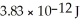 .What is the total change in mass (in kg)for this reaction?
.What is the total change in mass (in kg)for this reaction? 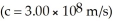
(Multiple Choice)
4.7/5  (35)
(35)
Which one of the following is a correct representation of a beta particle?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 21 - 40 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)