Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
List the common particles and their symbols used in descriptions of radioactive decay and nuclear transformations.
(Essay)
4.9/5  (28)
(28)
Gamma radiation only changes the atomic number but not the mass number of a nucleus.
(True/False)
4.8/5  (33)
(33)
What is the largest number of protons that can exist in a nucleus and still be stable?
(Multiple Choice)
4.9/5  (28)
(28)
The mass of a proton is 1.00728 amu and that of a neutron is 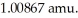 What is the binding energy
What is the binding energy 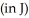 of a
of a 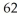 Co nucleus?
(The mass of a cobalt-62 nucleus is
Co nucleus?
(The mass of a cobalt-62 nucleus is 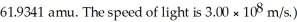
(Multiple Choice)
4.9/5  (38)
(38)
The missing product in this reaction combines with oxygen to form a compound with the formula ________.
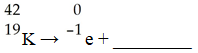
(Multiple Choice)
4.9/5  (33)
(33)
A rock contains 0.153 mg of lead-206 for each milligram of uranium-238.The  for the decay of uranium-238 to lead-206 is 4.5 × 109 yr.The rock was formed ________ years ago.
for the decay of uranium-238 to lead-206 is 4.5 × 109 yr.The rock was formed ________ years ago.
(Multiple Choice)
4.9/5  (36)
(36)
The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu.What is the binding energy (in J)of a 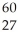 Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)
Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.Speed of light = 3.00 × 108 m/s.)
(Multiple Choice)
4.8/5  (41)
(41)
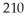 Pb has a half-life of 22.3 years and decays to produce 206Hg.If you start with 7.50 g of 210Pb,how many grams of 206Hg will you have after 17.5 years?
Pb has a half-life of 22.3 years and decays to produce 206Hg.If you start with 7.50 g of 210Pb,how many grams of 206Hg will you have after 17.5 years?
(Multiple Choice)
4.8/5  (29)
(29)
The carbon-14 dating method can be used to determine the age of a ________.
(Multiple Choice)
4.8/5  (36)
(36)
41Ca decays by electron capture.The product of this reaction undergoes alpha decay.What is the product of this second decay reaction?
(Multiple Choice)
4.8/5  (35)
(35)
The half-life of 131I is 0.220 years.How much of a 500.0 mg sample remains after 24 hours?
(Multiple Choice)
4.9/5  (37)
(37)
Positron emission causes an increase of one in the atomic number.
(True/False)
4.8/5  (41)
(41)
This reaction is an example of ________. 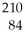 Po →
Po → 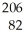 Pb + ________
Pb + ________
(Multiple Choice)
4.9/5  (28)
(28)
Showing 41 - 60 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)