Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The reaction shown below is responsible for creating 14C in the atmosphere.What is the bombarding

(Multiple Choice)
4.8/5  (27)
(27)
What happens to the mass number and the atomic number of an element when it undergoes alpha decay?
(Multiple Choice)
4.8/5  (41)
(41)
Transuranium elements have atomic numbers greater than ________.
(Multiple Choice)
4.9/5  (35)
(35)
If we start with 1.000 g of strontium-90,0.805 g will remain after 9.00 yr.This means that the 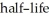 of strontium-90 is ________ yr.
of strontium-90 is ________ yr.
(Multiple Choice)
4.9/5  (33)
(33)
When an isotope undergoes electron capture,what happens to the captured electron?
(Essay)
4.8/5  (22)
(22)
Which one of the following natural radionuclides is the most abundant?
(Multiple Choice)
4.8/5  (34)
(34)
The product of the nuclear reaction in which 28Si is subjected to neutron capture followed by alpha emission is ________.
(Multiple Choice)
4.7/5  (36)
(36)
What happens to the atomic mass number and the atomic number of a radioisotope when it undergoes alpha emission?
(Essay)
4.8/5  (41)
(41)
Who is credited with first achieving fission of uranium-235?
(Multiple Choice)
4.9/5  (32)
(32)
The alpha decay of what isotope of what element produces lead-206?
(Multiple Choice)
4.8/5  (34)
(34)
The neutron/proton ratio of stable nuclei increases with increasing atomic number.
(True/False)
4.7/5  (32)
(32)
Which type of radioactive decay results in no change in mass number and atomic number for the starting nucleus?
(Multiple Choice)
4.8/5  (35)
(35)
The half-life of 218Po is 3.1 minutes.How much of a 155 gram sample remains after 0.40 hours?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 61 - 80 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)