Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The missing product in this reaction would be found in which group of the periodic table? 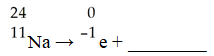
(Multiple Choice)
4.9/5  (39)
(39)
Alpha decay produces a new nucleus whose ________ than those respectively of the original nucleus.
(Multiple Choice)
4.8/5  (33)
(33)
Nuclei above the belt of stability can lower their neutron-to-proton ratio by ________.
(Multiple Choice)
4.8/5  (37)
(37)
The half-life of cobalt-60 is 5.20 yr.How many milligrams of a 2.000-mg sample remain after 9.50 years?
(Multiple Choice)
4.8/5  (20)
(20)
Bombardment of uranium-238 with a deuteron (hydrogen-2)generates neptunium-237 and ________ neutrons.
(Multiple Choice)
5.0/5  (36)
(36)
Which one of the following devices converts radioactive emissions to light for detection?
(Multiple Choice)
4.9/5  (34)
(34)
Radium undergoes alpha decay.The product of this reaction also undergoes alpha decay.What is the product of this second decay reaction?
(Multiple Choice)
4.9/5  (35)
(35)
In what type of radioactive decay does the atomic number of the product increase by one?
(Multiple Choice)
4.9/5  (33)
(33)
In the nuclear transmutation represented by
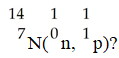 ,what is the emitted particle?
,what is the emitted particle?
(Multiple Choice)
4.8/5  (33)
(33)
What happens in the nucleus of an atom that undergoes positron emission?
(Essay)
4.8/5  (32)
(32)
Due to the nature of the positron,________ is actually detected in positron emission tomography.
(Multiple Choice)
4.8/5  (36)
(36)
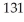 I has a half-life of 8.04 days.Assuming you start with a 1.03 mg sample of
I has a half-life of 8.04 days.Assuming you start with a 1.03 mg sample of  I,how many mg will remain after 13.0 days?
I,how many mg will remain after 13.0 days?
(Multiple Choice)
4.8/5  (38)
(38)
Atoms with the same atomic number and different mass numbers ________.
(Multiple Choice)
4.9/5  (33)
(33)
The initial element used to make cobalt-60 for cancer radiation therapy is ________.
(Short Answer)
4.8/5  (34)
(34)
Showing 161 - 178 of 178
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)