Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
What is the effect of adding NaOH(aq)to an aqueous solution of ammonia?
1)The pH of the solution will increase.
2)The concentration of NH4+(aq)will decrease.
3)The concentration of NH3(aq)will increase.
(Multiple Choice)
5.0/5  (27)
(27)
What is the molar solubility of silver(I)bromide at 25°C? The solubility product constant for silver(I)bromide is 5.0 10-13 at 25°C.
(Multiple Choice)
4.7/5  (45)
(45)
Suppose 50.00 mL of 2.0 10-6 M Fe(NO3)3 is added to 50.00 mL of 2.0 10-4 M KIO3.Which of the following statements is true? For Fe(IO3)3,Ksp = 1.0 10-14.
(Multiple Choice)
4.9/5  (48)
(48)
What is the minimum mass of Cs2CO3 (molar mass = 325.821 g/mol)that must be added to 54.8 mL of a 5.0 10-4 M AgNO3 solution in order for precipitation to occur? The Ksp of Ag2CO3 is 8.6 10-12.Assume no volume change occurs upon addition of Cs2CO3.
(Multiple Choice)
4.9/5  (31)
(31)
Given the following equilibrium constants,
Cd(IO3)2 Ksp = 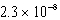 Cd(NH3)42+ Kf =
Cd(NH3)42+ Kf = 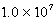 Determine K for the dissolution of the sparingly soluble salt Cd(IO3)2 in aqueous ammonia (shown below).Cd(IO3)2(s)+ 4NH3(aq)
Determine K for the dissolution of the sparingly soluble salt Cd(IO3)2 in aqueous ammonia (shown below).Cd(IO3)2(s)+ 4NH3(aq)  Cd(NH3)42+(aq)+ 2IO3-(aq)
Cd(NH3)42+(aq)+ 2IO3-(aq)
(Multiple Choice)
4.9/5  (32)
(32)
An aqueous solution contains 0.010 M Br- and 0.010 M I-.If Ag+ is added until AgBr(s)just begins to precipitate,what are the concentrations of Ag+ and I-? (Ksp of AgBr = 5.4 10-13,Ksp of AgI = 8.5 10-17)
(Multiple Choice)
4.7/5  (37)
(37)
A 25.00-mL sample of propionic acid,HC3H5O2,of unknown concentration was titrated with 0.143 M KOH.The equivalence point was reached when 43.76 mL of base had been added.What was the original concentration of the propionic acid?
(Multiple Choice)
4.9/5  (36)
(36)
The Ksp of BaSO4 is 1.1 10-10 at 25 C.What mass of BaSO4 (molar mass = 233.4 g/mol)will dissolve in 1.0 L of water at 25 C?
(Multiple Choice)
4.8/5  (41)
(41)
What is the molar solubility of Mn(OH)2(s)in a solution that is buffered at pH 8.00 at 25 C? The Ksp of Mn(OH)2 is 1.9 10-13 at 25 C.
(Multiple Choice)
4.9/5  (32)
(32)
What is the concentration of silver(I)ion in a saturated solution of silver(I)carbonate containing 0.0024 M Na2CO3? For Ag2CO3,Ksp = 8.6 10-12.
(Multiple Choice)
4.8/5  (27)
(27)
What is the pH of a buffer that results when 0.40 mol NaHCO2 is mixed with 100.0 mL of 2.00 M HCl(aq)and diluted with water to 250 mL? (Ka of HCO2H = 1.8 10-4)
(Multiple Choice)
4.7/5  (36)
(36)
What is the minimum concentration of Cu2+ required to begin precipitating Cu(OH)2(s)in a solution buffered at pH 10.63? The Ksp of Cu(OH)2 is 2.6 10-19 and Kw = 1.01 10-14.
(Multiple Choice)
4.9/5  (32)
(32)
Which acid-base combination is depicted by this titration curve? The dot on the curve is located at the titrant volume where the titration solution pH equals 7. 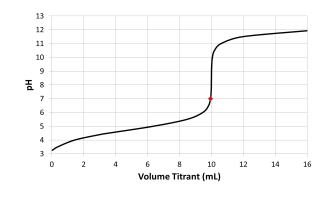
(Multiple Choice)
4.7/5  (42)
(42)
To make a buffer with a pH of 8.00,you should use a weak acid with a Ka close to _______.
(Essay)
4.8/5  (42)
(42)
An impure sample of sodium carbonate,Na2CO3,is titrated with 0.113 M HCl according to the reaction below.
2 HCl(aq)+ Na2CO3(aq) 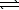 CO2(g)+ H2O(
CO2(g)+ H2O( 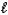 )+ 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
)+ 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
(Multiple Choice)
4.8/5  (38)
(38)
How many moles of solid NaF would have to be added to 1.0 L of 2.39 M HF solution to achieve a buffer of pH 3.35? Assume there is no volume change.(Ka for HF = 7.2 10-4)
(Multiple Choice)
4.8/5  (38)
(38)
A 50.00-mL solution of 0.0729 M chloroacetic acid (Ka = 1.4 10-3)is titrated with a 0.0181 M solution of NaOH as the titrant.What is the pH of at the equivalence point? (Kw = 1.00 10-14)
(Multiple Choice)
4.8/5  (40)
(40)
What is the pH of a solution made by combining 175 mL of 0.33 M NaC2H3O2 with 126 mL of 0.48 M HC2H3O2? The Ka of acetic acid is 1.75 10-5.
(Multiple Choice)
4.7/5  (38)
(38)
An acid-base equilibrium system is created by dissolving 0.50 mol CH3CO2H in water to a volume of 1.0 L.What is the effect of adding 0.50 mol CH3CO2-(aq)to this solution?
1)The pH of the solution will equal 7.00 because equal concentrations of a weak acid and its conjugate base are present.
2)Some CH3CO2H(aq)will ionize,increasing the concentration of CH3CO2-(aq)and increasing the pH.
3)Some CH3CO2-(aq)will react with H3O+,increasing the concentration of CH3CO2H(aq)and reestablishing the solution equilibrium.
(Multiple Choice)
4.8/5  (32)
(32)
Showing 21 - 40 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)