Exam 4: Three Major Classes of Chemical Reactions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Select the classification for the following reaction.
2NaCl(l) 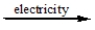 2Na(l)+ Cl2(g)
2Na(l)+ Cl2(g)
(Multiple Choice)
4.7/5  (24)
(24)
The oxidation numbers of P,S and Cl in H2PO2-,H2S and KClO4 are,respectively
(Multiple Choice)
4.9/5  (39)
(39)
In both of the following reactions,a precipitate is formed.Complete and balance the equations,showing the phases of the products.
a.BaCl2(aq)+ Na2SO4(aq)
b.Mg(NO3)2(aq)+ KOH(aq)
(Essay)
4.7/5  (43)
(43)
In a redox reaction,the reducing agent undergoes loss of electrons.
(True/False)
5.0/5  (34)
(34)
Calculate the oxidation number of sulfur in sodium metabisulfite,Na2S2O5.
(Multiple Choice)
4.8/5  (34)
(34)
Select the classification for the following reaction.
KOH(aq)+ HCl(aq) KCl(aq)+ H2O(l)
(Multiple Choice)
4.8/5  (36)
(36)
a.Explain or define what is meant by the term "oxidation".
b.Write down the oxidation numbers of the atoms in the following formulas.

(Essay)
4.8/5  (29)
(29)
In a redox reaction,the oxidizing agent undergoes loss of electrons.
(True/False)
4.8/5  (36)
(36)
Select the classification for the following reaction.
CaCl2·H2O(s) 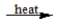 CaCl2(s)+ H2O(g)
CaCl2(s)+ H2O(g)
(Multiple Choice)
4.9/5  (36)
(36)
Predict the products by completing a balanced equation for the following decomposition reaction.
CaCl2(l) 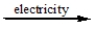 ?
?
(Multiple Choice)
4.9/5  (33)
(33)
Select the net ionic equation for the reaction between lithium hydroxide and hydrobromic acid.
LiOH(aq)+ HBr(aq) H2O(l)+ LiBr(aq)
(Multiple Choice)
5.0/5  (32)
(32)
In both of the following reactions,a precipitate is formed.Complete and balance the equations,showing the phases of the products.
a.AgNO3(aq)+ CaCl2(aq)
b.NaOH(aq)+ Fe(NO3)3(aq)
(Essay)
4.9/5  (39)
(39)
Automobile batteries use 3.0 M H2SO4 as an electrolyte.How much 1.20 M NaOH will be needed to neutralize 225 mL of battery acid?
H2SO4(aq)+ 2NaOH(aq) 2H2O(l)+ Na2SO4(aq)
(Multiple Choice)
4.8/5  (35)
(35)
Select the classification for the following reaction.
2H2O2(aq) 2H2O(l)+ O2(g)
(Multiple Choice)
4.9/5  (36)
(36)
In each of the following cases,write down the oxidation number of the indicated atom.
a.P in P4
b.C in C2H6
c.S in H2SO4
d.Mn in MnO4-
e.S in S4O62-
f.P in Na3PO4
(Essay)
4.8/5  (34)
(34)
Vinegar is a solution of acetic acid,CH3COOH,dissolved in water.A 5.54-g sample of vinegar was neutralized by 30.10 mL of 0.100 M NaOH.What is the percent by weight of acetic acid in the vinegar?
(Multiple Choice)
4.9/5  (28)
(28)
Showing 61 - 80 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)