Exam 7: Quantum Theory and Atomic Structure
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Contact lenses can focus light due to the ___________ of the waves.
(Multiple Choice)
4.9/5  (40)
(40)
Use the Rydberg equation to calculate the wavelength,in nm,of the least energetic (longest wavelength)line in the visible series (n1 = 2)of the spectrum of atomic hydrogen.
(Short Answer)
4.9/5  (36)
(36)
The following combinations of quantum numbers are not allowed.Correct each set by changing only one quantum number,and write in an appropriate corrected value.
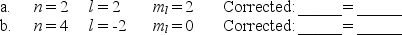
(Short Answer)
4.8/5  (43)
(43)
In the quantum mechanical treatment of the hydrogen atom,the energy depends on the principal quantum number n but not on the values of l or ml.
(True/False)
4.8/5  (31)
(31)
a.Calculate the wavelength in nm of a photon whose energy is 6.00 10-19 J.
b.Would the photon in (a)have enough energy to ionize a hydrogen atom in its ground state (i.e. ,to separate the proton and electron completely)? Use the Bohr equation to explain your answer.
(Essay)
4.8/5  (31)
(31)
Explain the context and meanings of the terms "orbit" and "orbital",making a clear distinction between them.
(Essay)
4.8/5  (35)
(35)
a.Use the Bohr equation to calculate the energy needed to ionize a hydrogen atom from its ground state.
b.What is the minimum wavelength of a photon needed for it to have the energy needed in (a)?
(Essay)
4.8/5  (33)
(33)
Use the Bohr equation to calculate the energy of
a.the largest energy absorption or emission process involving the n = 2 state of the hydrogen atom.
b.the smallest energy absorption or emission process involving the n = 2 state of the hydrogen atom.
(Essay)
4.9/5  (35)
(35)
Who was the first scientist to propose that the atom had a dense nucleus which occupied only a small fraction of the volume of the atom?
(Multiple Choice)
4.8/5  (30)
(30)
a.Use Bohr's equation to calculate how much energy (in J)is needed to promote an electron from the H-atom ground state to the n = 4 level.
b.If a photon provides the energy in (a),what is its wavelength in nm?
(Essay)
4.7/5  (31)
(31)
For the following orbitals,state the values of n,l and ml which apply,and draw a sketch showing the shape and orientation of the orbital.
a.3s
b.2px
(Essay)
4.8/5  (35)
(35)
Line spectra from all regions of the electromagnetic spectrum,including the Paschen series of infrared lines for hydrogen,are used by astronomers to identify elements present in the atmospheres of stars.Calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3.(R = 1.096776 107 m-1)
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following is a correct set of quantum numbers for an electron in a 5f orbital?
(Multiple Choice)
4.9/5  (33)
(33)
A photon has an energy of 5.53 10-17 J.What is its frequency in s-1?
(Multiple Choice)
4.9/5  (39)
(39)
Who was the first scientist to propose that an object could emit only certain amounts of energy?
(Multiple Choice)
5.0/5  (38)
(38)
The energy of an electron in the hydrogen atom is determined by
(Multiple Choice)
4.9/5  (43)
(43)
What are the possible values for the following quantum numbers in an atom?
a.n
b.l
c.ml
(Essay)
4.8/5  (37)
(37)
What is the speed of an electron in m/s if its wavelength is 0.155 nm?
(Essay)
4.9/5  (30)
(30)
The AM station KBOR plays your favorite music from the 20s and 30s at 1290 kHz.Find the wavelength of these waves.
(Multiple Choice)
4.8/5  (27)
(27)
Showing 21 - 40 of 72
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)