Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
For a chemical reaction to be spontaneous at all temperatures,which of the following conditions must be met?
(Multiple Choice)
4.9/5  (41)
(41)
Calculate S° for the reaction
2Cl2(g)+ SO2(g) SOCl2(g)+ Cl2O(g)
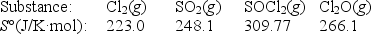
(Multiple Choice)
4.9/5  (48)
(48)
Iron(III)oxide can be reduced by carbon monoxide.
Fe2O3(s)+ 3CO(g)  2Fe(s)+ 3CO2(g)
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature.
2Fe(s)+ 3CO2(g)
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature.
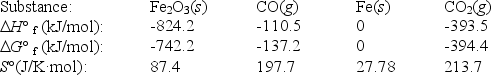
(Multiple Choice)
4.9/5  (40)
(40)
For each of the following pairs,predict which (A or B)will have the greater entropy,and in one sentence indicate your reasoning.
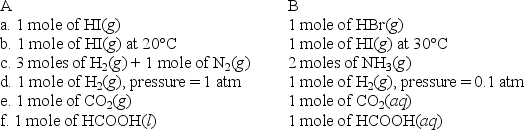
(Essay)
4.7/5  (33)
(33)
Which relationship best describes S° for the following reaction?
CO(g)+ H2O(g) CO2(g)+ H2(g)
(Multiple Choice)
4.8/5  (39)
(39)
You are given pure samples of pentane,CH3CH2CH2CH2CH3(l),and 1,3-pentadiene,CH2=CHCH=CHCH3(l).What prediction would you make concerning the standard molar entropies of pentane,S°(pentane)and 1,3-pentadiene,S°(1,3-pentadiene),at 298 K?
(Multiple Choice)
4.9/5  (39)
(39)
The term microstate refers to the energy state of a single molecule in a system of many molecules.
(True/False)
4.7/5  (44)
(44)
The reaction of methane with water to form carbon dioxide and hydrogen is non-spontaneous at 298 K.At what temperature will this system make the transition from non-spontaneous to spontaneous? The data refer to 298 K.
CH4(g)+ 2H2O(g)  CO2(g)+ 4H2(g)
CO2(g)+ 4H2(g)

(Multiple Choice)
4.9/5  (43)
(43)
Under a given set of conditions,all microstates of a system are equally probable.
(True/False)
5.0/5  (36)
(36)
Which of the following is always true for an exothermic process?
(Multiple Choice)
4.8/5  (36)
(36)
The formation constant for the reaction
Ag+(aq)+ 2NH3(aq)  Ag(NH3)2+(aq)
Is Kf = 1.7 107 at 25°C.What is G° at this temperature?
Ag(NH3)2+(aq)
Is Kf = 1.7 107 at 25°C.What is G° at this temperature?
(Multiple Choice)
4.7/5  (27)
(27)
Elemental boron can be formed by reaction of boron trichloride with hydrogen.
BCl3(g)+ 1.5H2(g) B(s)+ 3HCl(g)
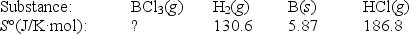 If S° = 80.3 J/K,what is S° for BCl3(g)?
If S° = 80.3 J/K,what is S° for BCl3(g)?
(Multiple Choice)
4.8/5  (33)
(33)
The complete combustion of liquid benzene is represented by the equation:
C6H6(l)+ 7 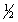 O2(g) 6CO2(g)+ 3H2O(l)
Using the data below,calculate,for this reaction
a. H°
b. S°
c. G° at 25°C.
O2(g) 6CO2(g)+ 3H2O(l)
Using the data below,calculate,for this reaction
a. H°
b. S°
c. G° at 25°C.
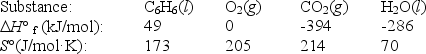
(Essay)
4.8/5  (37)
(37)
In the expression,S = k ln W,W is called the number of microstates.Explain clearly the meaning of the word "microstate",and why a system under a given set of conditions normally has many microstates.
(Essay)
4.9/5  (42)
(42)
A chemical reaction has G° = 10.0 kJ and S° = 50.0 J/K
a.Calculate H° for this reaction at 25°C.
b.Could this reaction ever be spontaneous? Explain your answer.
(Essay)
4.9/5  (35)
(35)
What is the free energy change, G°,for the equilibrium between hydrogen iodide,hydrogen,and iodine at 453°C? Kc = 0.020
2HI(g)  H2(g)+ I2(g)
H2(g)+ I2(g)
(Multiple Choice)
4.8/5  (33)
(33)
Given: C2H2(g) 2C(graphite)+ H2(g) G° = -209 kJ
A sample of gaseous C2H2 (acetylene,or ethyne)was stored for one year,yet at the end of this period the sample remained unchanged and no graphite or hydrogen gas had been formed.Briefly explain why there is no inconsistency between the sign of G° and the apparent stability of the sample.
(Essay)
4.8/5  (51)
(51)
Which relationship or statement best describes S° for the following reaction?
C2H5OH(l)+ 3O2(g) 2CO2(g)+ 3H2O(l)
(Multiple Choice)
4.8/5  (35)
(35)
Which relationship or statement best describes S° for the following reaction?
2NH3(g)+ 2ClF3(g) 6HF(g)+ N2(g)+ Cl2(g)
(Multiple Choice)
4.8/5  (40)
(40)
In which one of the following pairs will the first system have a higher entropy than the second? Assume P and T are the same for each pair,unless stated otherwise.
(Multiple Choice)
4.9/5  (44)
(44)
Showing 41 - 60 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)