Exam 23: The Transition Elements and Their Coordination Compounds
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar). 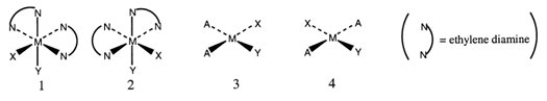 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
(Multiple Choice)
4.9/5  (32)
(32)
If M represents a transition element, which of the following oxides should be the least basic?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following transition elements can have an oxidation number of +7?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following ions could exist in only the high-spin state in an octahedral complex?
(Multiple Choice)
4.9/5  (36)
(36)
The compound K3[Fe(CN)6] is used in calico printing and wool dyeing. Give its systematic name.
(Multiple Choice)
4.8/5  (35)
(35)
What is the highest possible oxidation state for molybdenum, Mo?
(Multiple Choice)
4.9/5  (40)
(40)
According to valence bond theory, what would be the set of hybrid orbitals used when a Period 4 transition metal forms a square planar complex?
(Multiple Choice)
4.9/5  (40)
(40)
According to Valence Bond theory, in the square planar Ni(CN)42- complex ion, the orbital hybridization pattern is
(Multiple Choice)
4.8/5  (34)
(34)
According to valence bond theory, what would be the set of hybrid orbitals used when a Period 4 transition metal forms a tetrahedral complex?
(Multiple Choice)
4.9/5  (37)
(37)
In the spectrochemical series, which one of the following ligands has the strongest field?
(Multiple Choice)
4.8/5  (38)
(38)
Aluminum reacts with oxygen in the air to form a protective oxide coating. Silver also reacts with compounds in air to form a black coating. What substance is formed?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following ions is most likely to form colored compounds?
(Multiple Choice)
4.9/5  (35)
(35)
All atoms of the first transition series of elements have the ground state electronic configuration [Ar]4s23dx, where x is an integer from 1 to 10.
(True/False)
4.9/5  (24)
(24)
The dxy and the dx2-y2 orbitals both lie in the xy plane, yet for a metal ion in an octahedral complex the energy of the dxy orbital is lower than that of the dx2-y2orbital. Explain this using the arguments of crystal field theory.
(Essay)
4.7/5  (32)
(32)
The most common oxidation state for ions of the transition elements is
(Multiple Choice)
4.9/5  (37)
(37)
Showing 41 - 60 of 92
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)