Exam 39: Quantum Mechanics
Exam 1: Units, Physical Quantities, and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum, Impulse, and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Equilibrium and Elasticity50 Questions
Exam 11: Fluid Mechanics50 Questions
Exam 12: Gravitation50 Questions
Exam 13: Periodic Motion50 Questions
Exam 14: Mechanical Waves44 Questions
Exam 15: Sound and Hearing66 Questions
Exam 16: Temperature and Heat63 Questions
Exam 17: Thermal Properties of Matter58 Questions
Exam 18: The First Law of Thermodynamics52 Questions
Exam 19: The Second Law of Thermodynamics50 Questions
Exam 20: Electric Charge and Electric Field58 Questions
Exam 21: Gausss Law41 Questions
Exam 22: Electric Potential55 Questions
Exam 23: Capacitance and Dielectrics52 Questions
Exam 24: Current, Resistance, and Electromotive Force50 Questions
Exam 25: Direct-Current Circuits53 Questions
Exam 26: Magnetic Field and Magnetic Forces36 Questions
Exam 27: Sources of Magnetic Field51 Questions
Exam 28: Electromagnetic Induction39 Questions
Exam 29: Inductance26 Questions
Exam 30: Alternating Current49 Questions
Exam 31: Electromagnetic Waves47 Questions
Exam 32: The Nature and Propagation of Light28 Questions
Exam 33: Geometric Optics81 Questions
Exam 34: Interference33 Questions
Exam 35: Diffraction49 Questions
Exam 36: Relativity51 Questions
Exam 37: Photons: Light Waves Behaving As Particles38 Questions
Exam 38: Particles Behaving As Waves52 Questions
Exam 39: Quantum Mechanics40 Questions
Exam 40: Atomic Structure41 Questions
Exam 41: Molecules and Condensed Matter31 Questions
Exam 42: Nuclear Physics89 Questions
Exam 43: Particle Physics and Cosmology44 Questions
Select questions type
An electron is confined in a harmonic oscillator potential well. A photon is emitted when the electron undergoes a 3→1 quantum jump. What is the wavelength of the emission if the net force on the electron behaves as though it has a spring constant of 9.6 N/m? (mel = 9.11 × 10-31 kg, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, 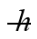 = 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
= 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
Free
(Multiple Choice)
4.7/5  (35)
(35)
Correct Answer:
A
One fairly crude method of determining the size of a molecule is to treat the molecule as an infinite square well (a box) with an electron trapped inside, and to measure the wavelengths of emitted photons. If the photon emitted during the n = 2 to n = 1 transition has wavelength 1940 nm, what is the width of the molecule? (c = 3.00 × 108 m/s, h = 6.626 × 10-34J ∙ s, mel = 9.11 × 10-31 kg)
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
A
An electron is confined in a one-dimensional box (an infinite well). Two adjacent allowed energies of the electron are 1.068 × 10-18 J and 1.352 × 10-18 J. What is the length of the box? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
A 3.10-eV electron is incident on a 0.40-nm barrier that is 5.67 eV high. What is the probability that this electron will tunnel through the barrier? (1 eV = 1.60 × 10-19 J, mel = 9.11 × 10-31 kg, h = 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.9/5  (39)
(39)
The square of the wave function of a particle, |ψ(x)|2, gives the probability of finding the particle at the point x.
(True/False)
4.9/5  (30)
(30)
A particle is confined to a one-dimensional box (an infinite well) on the x-axis between x = 0 and x = L. The potential height of the walls of the box is infinite. The normalized wave function of the particle, which is in the ground state, is given by ψ(x) = 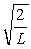 sin
sin 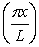 , with 0 ≤ x ≤ L. What is the probability of finding the particle between x = 0 and x = L/3?
, with 0 ≤ x ≤ L. What is the probability of finding the particle between x = 0 and x = L/3?
(Multiple Choice)
4.9/5  (36)
(36)
An electron is in an infinite square well (a box) that is 2.0 nm wide. The electron makes a transition from the n = 8 to the n = 7 state, what is the wavelength of the emitted photon? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19)
(Multiple Choice)
4.8/5  (28)
(28)
The energy of a proton is 1.0 MeV below the top of a 1.2-MeV-high energy barrier that is 6.8 fm wide. What is the probability that the proton will tunnel through the barrier? (1 eV = 1.60 × 10-19 J, mproton = 1.67 × 10-27 kg, 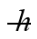 = 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
= 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.9/5  (40)
(40)
The atoms in a nickel crystal vibrate as harmonic oscillators with an angular frequency of 2.3 × 1013 rad/s. The mass of a nickel atom is 9.75 × 10-26 kg. What is the difference in energy between adjacent vibrational energy levels of nickel? (h = 6.626 × 10-34 J ∙ s, 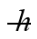 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (39)
(39)
An electron is trapped in an infinite square well (a box) of width 6.88 nm. Find the wavelength of photons emitted when the electron drops from the n = 5 state to the n = 1 state in this system. (c = 3.00 × 108 m/s, h = 6.626 × 10-34J ∙ s, mel = 9.11 × 10-31 kg)
(Multiple Choice)
4.8/5  (25)
(25)
The wave function for an electron that is confined to x ≥ 0 nm is
ψ(x) = 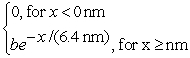 (a) What must be the value of b?
(b) What is the probability of finding the electron in a 0.010 nm-wide region centered at x = 1.0 nm?
(a) What must be the value of b?
(b) What is the probability of finding the electron in a 0.010 nm-wide region centered at x = 1.0 nm?
(Short Answer)
4.8/5  (31)
(31)
The wave function for a particle must be normalizable because
(Multiple Choice)
4.9/5  (39)
(39)
An electron is in the ground state of an infinite well (a box) where its energy is 5.00 eV. In the next higher level, what would its energy be? (1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (33)
(33)
An electron in an infinite potential well (a box) makes a transition from the n = 3 level to the ground state and in so doing emits a photon of wavelength 20.9 nm. (c = 3.00 × 10⁸ m/s, h = 6.626 × 10⁻³⁴J ∙ s, mel = 9.11 × 10⁻³¹ kg)
(a) What is the width of this well?
(b) What wavelength photon would be required to excite the electron from its original level to the next higher one?
(Short Answer)
4.8/5  (47)
(47)
The lowest energy level of a particle confined to a one-dimensional region of space (a box, or infinite well) with fixed length L is E0. If an identical particle is confined to a similar region with fixed length L/6, what is the energy of the lowest energy level that the particles have in common? Express your answer in terms of E0.
(Short Answer)
4.8/5  (37)
(37)
You want to have an electron in an energy level where its speed is no more than 66 m/s. What is the length of the smallest box (an infinite well) in which you can do this? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg)
(Multiple Choice)
5.0/5  (36)
(36)
If an atom in a crystal is acted upon by a restoring force that is directly proportional to the distance of the atom from its equilibrium position in the crystal, then it is impossible for the atom to have zero kinetic energy.
(True/False)
4.8/5  (30)
(30)
The smallest kinetic energy that an electron in a box (an infinite well) can have is zero.
(True/False)
4.9/5  (33)
(33)
An electron is in an infinite square well that is 2.6 nm wide. What is the smallest value of the state quantum number n for which the energy level exceeds 100 eV? (h = 6.626 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19)
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the ground state energy of a harmonic oscillator with a classical frequency of 3.68 × 1015 Hz. (1 eV = 1.60 × 10-19 J, h = 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.9/5  (40)
(40)
Showing 1 - 20 of 40
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)