Exam 41: Molecules and Condensed Matter
Exam 1: Units, Physical Quantities, and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum, Impulse, and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Equilibrium and Elasticity50 Questions
Exam 11: Fluid Mechanics50 Questions
Exam 12: Gravitation50 Questions
Exam 13: Periodic Motion50 Questions
Exam 14: Mechanical Waves44 Questions
Exam 15: Sound and Hearing66 Questions
Exam 16: Temperature and Heat63 Questions
Exam 17: Thermal Properties of Matter58 Questions
Exam 18: The First Law of Thermodynamics52 Questions
Exam 19: The Second Law of Thermodynamics50 Questions
Exam 20: Electric Charge and Electric Field58 Questions
Exam 21: Gausss Law41 Questions
Exam 22: Electric Potential55 Questions
Exam 23: Capacitance and Dielectrics52 Questions
Exam 24: Current, Resistance, and Electromotive Force50 Questions
Exam 25: Direct-Current Circuits53 Questions
Exam 26: Magnetic Field and Magnetic Forces36 Questions
Exam 27: Sources of Magnetic Field51 Questions
Exam 28: Electromagnetic Induction39 Questions
Exam 29: Inductance26 Questions
Exam 30: Alternating Current49 Questions
Exam 31: Electromagnetic Waves47 Questions
Exam 32: The Nature and Propagation of Light28 Questions
Exam 33: Geometric Optics81 Questions
Exam 34: Interference33 Questions
Exam 35: Diffraction49 Questions
Exam 36: Relativity51 Questions
Exam 37: Photons: Light Waves Behaving As Particles38 Questions
Exam 38: Particles Behaving As Waves52 Questions
Exam 39: Quantum Mechanics40 Questions
Exam 40: Atomic Structure41 Questions
Exam 41: Molecules and Condensed Matter31 Questions
Exam 42: Nuclear Physics89 Questions
Exam 43: Particle Physics and Cosmology44 Questions
Select questions type
A rotating diatomic molecule has rotational quantum number l. The energy DIFFERENCE between adjacent energy levels
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
A
The vibrational frequency of an HF molecule is 8.72 × 1013 Hz and the reduced mass of the molecule is 1.589 × 10-27 kg. What is the ground state vibrational energy of an HF molecule? (1 eV = 1.60 × 10-19 J, 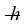 = 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
= 1.055 × 10-34 J ∙ s, h = 6.626 × 10-34 J ∙ s)
Free
(Multiple Choice)
4.9/5  (42)
(42)
Correct Answer:
B
A diatomic molecule has 18 × 10-5 eV of rotational energy in the l = 2 quantum state. What is its rotational energy in the l = 0 quantum state?
(Multiple Choice)
4.7/5  (36)
(36)
A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state. What is the frequency of vibration of the molecule? (h = 6.626 × 10-34 J ∙ s, 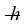 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (27)
(27)
The moment of inertia of a fluorine (F2) molecule is 3.167 × 10-46. What is the rotational energy of a fluorine molecule for the l = 19 state? (h = 6.626 × 10-34 J ∙ s, 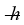 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (28)
(28)
A diatomic has a moment of inertia of 7.73 × 10-45 kg∙ m2. What is its rotational energy in the quantum state characterized by l = 2? (h = 6.626 × 10-34 J ∙ s, 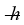 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (40)
(40)
A certain diatomic molecule emits a photon of energy 1.20 eV when it makes a transition from the n = 1 vibrational state to the next lower vibrational state. If the molecule made a transition from the n = 2 state to the n = 1 state, what would be the energy of the photon it would emit? (h = 6.626 × 10-34 J ∙ s, 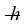 = 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (36)
(36)
Estimate the rotational energy (in eV) for a diatomic hydrogen molecule in the l = 2 quantum state. (The equilibrium separation for the H2 molecule is 0.074 nm.) (1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s, 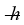 = 1.055 × 10-34 J ∙ s, mH ≈ mproton = 1.67 × 10-27 kg)
= 1.055 × 10-34 J ∙ s, mH ≈ mproton = 1.67 × 10-27 kg)
(Multiple Choice)
4.9/5  (47)
(47)
When a certain diatomic molecule undergoes a transition from the l = 5 to the l = 3 rotational level, the emitted photon has wavelength 2.87 × 10-4 m. Calculate the moment of inertia of the molecule. (c = 3.00 × 108 m/s, e = 1.60 × 10-19 C, h = 6.626 × 10-34 J ∙ s, 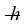 = 1.055 × 10-34 J ∙ s)
= 1.055 × 10-34 J ∙ s)
(Short Answer)
4.9/5  (35)
(35)
What is the occupancy probability at an energy of 12.00 eV for a material with a Fermi energy (level) of 11.63 eV at a temperature of 500 K? (mel = 9.11 × 10-31 kg, h = 6.626 × 10-34 J ∙ s, Boltzmann constant = 1.38 × 10-23, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (32)
(32)
Approximately how many states in the range from 5.0 eV to 5.2 eV are there in a copper bar of volume 5.3 cm3? (h = 6.626 × 10-34 J ∙ s, 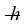 = 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19 J)
= 1.055 × 10-34 J ∙ s, mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (37)
(37)
A rotating diatomic molecule in its l = 1 quantum state has energy E. What is the energy of the same molecule in its l = 2 quantum state?
(Multiple Choice)
4.8/5  (22)
(22)
A metal has a Fermi level (energy) of 5.50 eV. At 1200 K, what energy will have a 90% occupancy probability? (mel = 9.11 × 10-31 kg, h = 6.626 × 10-34 J ∙ s, Boltzmann constant = 1.38 × 10-23, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (38)
(38)
A vibrating diatomic molecule has vibrational quantum number n. The energy DIFFERENCE between adjacent energy levels
(Multiple Choice)
4.8/5  (26)
(26)
The energy gap between the valence and conduction bands in a certain semiconductor is 1.25 eV. What is the threshold wavelength for optical absorption in this substance? (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.7/5  (35)
(35)
The Fermi level (energy) of a metal is 5.5 eV. What is the number of conduction electrons per unit volume for this metal? (mel = 9.11 × 10-31 kg, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
(Multiple Choice)
4.8/5  (29)
(29)
A vibrating diatomic molecule in its ground state has energy E. What is the energy of the same molecule in its second EXCITED state?
(Multiple Choice)
4.8/5  (28)
(28)
Showing 1 - 20 of 31
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)