Exam 6: An Introduction to Metabolism
Exam 1: Introduction: Evolution and the Foundations of Biology36 Questions
Exam 2: The Chemical Context of Life137 Questions
Exam 3: Carbon and the Molecular Diversity of Life136 Questions
Exam 4: A Tour of the Cell75 Questions
Exam 5: Membrane Transport and Cell Signaling97 Questions
Exam 6: An Introduction to Metabolism79 Questions
Exam 7: Cellular Respiration and Fermentation100 Questions
Exam 8: Photosynthesis72 Questions
Exam 9: The Cell Cycle56 Questions
Exam 10: Meiosis and Sexual Life Cycles62 Questions
Exam 11: Mendel and the Gene Idea63 Questions
Exam 12: The Chromosomal Basis of Inheritance46 Questions
Exam 13: The Molecular Basis of Inheritance67 Questions
Exam 14: Gene Expression: From Gene to Protein80 Questions
Exam 15: Regulation of Gene Expression50 Questions
Exam 16: Development, Stem Cells, and Cancer34 Questions
Exam 17: Viruses35 Questions
Exam 18: Genomes and Their Evolution29 Questions
Exam 19: Descent With Modification55 Questions
Exam 20: Phylogeny60 Questions
Exam 21: The Evolution of Populations70 Questions
Exam 22: The Origin of Species67 Questions
Exam 23: Broad Patterns of Evolution45 Questions
Exam 24: Early Life and the Diversification of Prokaryotes88 Questions
Exam 25: The Origin and Diversification of Eukaryotes71 Questions
Exam 26: The Colonization of Land by Plants and Fungi126 Questions
Exam 27: The Rise of Animal Diversity88 Questions
Exam 28: Plant Structure and Growth59 Questions
Exam 29: Resource Acquisition, Nutrition, and Transport in Vascular Plants110 Questions
Exam 30: Reproduction and Domestication of Flowering Plants67 Questions
Exam 31: Plant Responses to Internal and External Signals75 Questions
Exam 32: Homeostasis and Endocrine Signaling120 Questions
Exam 33: Animal Nutrition67 Questions
Exam 34: Circulation and Gas Exchange88 Questions
Exam 35: The Immune System91 Questions
Exam 36: Reproduction and Development118 Questions
Exam 37: Neurons, Synapses, and Signaling76 Questions
Exam 38: Nervous and Sensory Systems99 Questions
Exam 39: Motor Mechanisms and Behavior79 Questions
Exam 40: Population Ecology and the Distribution of Organisms93 Questions
Exam 41: Species Interactions60 Questions
Exam 42: Ecosystems and Energy90 Questions
Exam 43: Global Ecology and Conservation Biology72 Questions
Select questions type
Which of the following types of reactions would decrease the entropy within a cell?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is a statement of the first law of thermodynamics?
(Multiple Choice)
4.9/5  (28)
(28)
Increasing the enzyme concentration in an enzymatic reaction could overcome which of the following?
(Multiple Choice)
4.9/5  (34)
(34)
The following question(s) are based on the reaction A + B ↔ C + D shown in Figure 6.4. 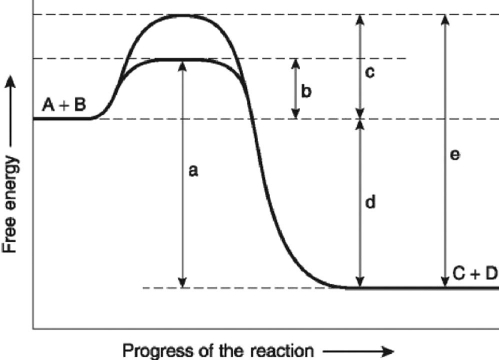 Figure 6.4
-Which of the following represents the activation energy required for a noncatalyzed reaction in Figure 6.4?
Figure 6.4
-Which of the following represents the activation energy required for a noncatalyzed reaction in Figure 6.4?
(Multiple Choice)
4.8/5  (36)
(36)
The following question(s) are based on the reaction A + B ↔ C + D shown in Figure 6.4.  Figure 6.4
-Which of the following represents the activation energy required for the enzyme-catalyzed reaction in Figure 6.4?
Figure 6.4
-Which of the following represents the activation energy required for the enzyme-catalyzed reaction in Figure 6.4?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following reactions tend to require an input of energy?
(Multiple Choice)
4.7/5  (31)
(31)
Which of the following describes the critical role that ATP plays in cellular metabolism?
(Multiple Choice)
4.9/5  (40)
(40)
In addition to regulating enzymes with activators and inhibitors, cells also regulate enzyme activity by
(Multiple Choice)
4.7/5  (28)
(28)
Which of the following is true of metabolism in its entirety in all organisms?
(Multiple Choice)
4.9/5  (27)
(27)
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following?
(Multiple Choice)
4.8/5  (37)
(37)
Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the second law of thermodynamics?
(Multiple Choice)
4.9/5  (41)
(41)
A number of systems for pumping ions across membranes are powered by ATP. Such ATP-powered pumps are often called ATPases, although they don't often hydrolyze ATP unless they are simultaneously transporting ions. Because small increases in calcium ions in the cytosol can trigger a number of different intracellular reactions, cells keep the cytosolic calcium concentration quite low under normal conditions, using ATP-powered calcium pumps. For example, muscle cells transport calcium from the cytosol into the membranous system called the sarcoplasmic reticulum (SR). If a resting muscle cell's cytosol has a free calcium ion concentration of 10-7 while the concentration in the SR is 10-2, then which of the following is the most likely mechanism by which the muscle cell ATPase maintains intracellular calcium concentrations?
(Multiple Choice)
4.7/5  (34)
(34)
Hydrolysis of ATP releases energy, which results in the production of ADP and inorganic phosphate. What is commonly the ultimate fate of inorganic phosphate produced in the cytosol?
(Multiple Choice)
4.8/5  (36)
(36)
The following question(s) are based on the reaction A + B ↔ C + D shown in Figure 6.4.  Figure 6.4
-Which of the following represents the activation energy needed for the enzyme-catalyzed reverse reaction, C + D → A + B, in Figure 6.4?
Figure 6.4
-Which of the following represents the activation energy needed for the enzyme-catalyzed reverse reaction, C + D → A + B, in Figure 6.4?
(Multiple Choice)
5.0/5  (25)
(25)
Some of the drugs used to treat HIV patients are competitive inhibitors of the HIV reverse transcriptase enzyme. Unfortunately, the high mutation rate of HIV means that the virus rapidly acquires mutations with amino acid changes that make them resistant to these competitive inhibitors. Where in the reverse transcriptase enzyme would such amino acid changes most likely occur in drug-resistant viruses?
(Multiple Choice)
4.8/5  (35)
(35)
The following question(s) are based on the reaction A + B ↔ C + D shown in Figure 6.4.  Figure 6.4
-Which of the following in Figure 6.4 would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
Figure 6.4
-Which of the following in Figure 6.4 would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
(Multiple Choice)
4.8/5  (30)
(30)
The cellular process of breaking down large molecules into smaller ones is defined as
(Multiple Choice)
4.9/5  (43)
(43)
Showing 41 - 60 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)